Clinical practice patterns in laryngeal cancer and introduction of CT lung screening
- PMID: 31005338
- PMCID: PMC6570546
- DOI: 10.1016/j.amjoto.2019.04.010
Clinical practice patterns in laryngeal cancer and introduction of CT lung screening
Abstract
Objectives: After the publication of large clinical trials, in January 2014 The U.S. Preventive Services Task Force (USPSTF) recommended annual lung cancer screening with low-dose CT in a well-defined group of high-risk smokers. A significant proportion of patients with laryngeal cancer (LC) meet the introduced criteria, and we hypothesized that clinical practice would change as a result of these evidence-based guidelines.
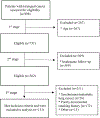
Methods: Retrospective chart review of patients diagnosed with LC and treated at Johns Hopkins Hospital who met USPSTF criteria for annual chest screening and were followed for at least 3 consecutive years in the years surrounding the introduction of screening guidelines (January 2010 to December 2017) was performed to identify those who had recommended screening CT chest.
Results: A total of 151 patients met the inclusion criteria of the study and were followed for a total of 746 patient-years. 184/332 (55%) patient-years in the pre-guidelines period and 246/414 (59%) in the post-guidelines period included at least one recommended chest imaging (CT or PET-CT; p = 0.27). 248/332 (75%) patient-years in the pre-guidelines period and 314/414 (76%) in the post-guidelines period included any radiological chest imaging (X-ray, CT or PET-CT; p = 0.72). Screening scans were ordered by OHNS (45%), Medical Oncology (31%), Radiation Oncology (8%), and primary care (14%) with 70% of patients missing at least one year of indicated screening.
Conclusions: The implementation of new lung cancer screening guidelines did not change clinical practice in the management of patients with LC and many patients do not receive recommended screening. Further study concerning potential barriers to effective evidence-based screening and coordination of care is warranted.
Keywords: CT; Head and neck cancer; Laryngeal cancer; Lung cancer; Lung cancer screening.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest: None of the authors have any conflicts of interest or financial ties to disclose.
The authors whose names are listed immediately above certify that they have NO affiliations or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Figures
References
-
- Global Burden of Disease Cancer Collaboration C, Fitzmaurice C, Allen C, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017;3(4):524–548. doi: 10.1001/jamaoncol.2016.5688 - DOI - PMC - PubMed
-
- Lewis A, Kang R, Levine A, Maghami E. The New Face of Head and Neck Cancer: The HPV Epidemic. Oncology (Williston Park). 2015;29(9):616–626. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

