Mind the gap: observation windows to define periods of event ascertainment as a quality control method for longitudinal electronic health record data
- PMID: 31005552
- PMCID: PMC6502687
- DOI: 10.1016/j.annepidem.2019.01.015
Mind the gap: observation windows to define periods of event ascertainment as a quality control method for longitudinal electronic health record data
Abstract
Purpose: Use of electronic health records (EHRs) in health research may lead to the false assumption of complete event ascertainment. We estimated "observation windows" (OWs), defined as periods within which the assumption of complete ascertainment of events is more likely to hold, as a quality control approach to reducing the likelihood of this false assumption. We demonstrated the impact of OWs on estimating the rates of type II diabetes mellitus (diabetes) from HIV clinical cohorts.
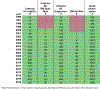
Methods: Data contributed by 16 HIV clinical cohorts to the NA-ACCORD were used to identify and evaluate OWs for an operationalized definition of diabetes occurrence as a case study. Procedures included (1) gathering cohort-level data; (2) visualizing and summarizing gaps in observations; (3) systematically establishing start and stop dates during which the assumption of complete ascertainment of diabetes events was reasonable; and (4) visualizing the diabetes OWs relative to the cohort open and close dates to identify immortal person-time. We estimated diabetes occurrence event rates and 95% confidence intervals in the most recent decade that data were available (January 1, 2007, to December 31, 2016).
Results: The number of diabetes events decreased by 17% with the use of the diabetes OWs; immortal person-time was removed decreasing total person-years by 23%. Consequently, the diabetes rate increased from 1.23 (95% confidence interval [1.20, 1.25]) per 100 person-years to 1.32 [1.29, 1.35] per 100 person-years with the use of diabetes OWs.
Conclusions: As the use of EHR-curated data for event-driven health research continues to expand, OWs have utility as a quality control approach to complete event ascertainment, helping to improve accuracy of estimates by removing immortal person-time when ascertainment is incomplete.
Keywords: Diabetes; Electronic medical records; HIV; Health research; Immortal person-time; Quality control.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of Interest:
KN Althoff serves on the Scientific Advisory Board for TrioHealth (outside the submitted work). MJ Silverberg received research grants to his institution from Pfizer and Merck (outside the submitted work).
There are no other conflicts of interest.
Figures





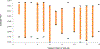
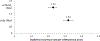
References
Publication types
MeSH terms
Grants and funding
- Z01 CP010176/ImNIH/Intramural NIH HHS/United States
- U01 AI031834/AI/NIAID NIH HHS/United States
- P30 AI094189/AI/NIAID NIH HHS/United States
- U01 AI038855/AI/NIAID NIH HHS/United States
- U54 MD007587/MD/NIMHD NIH HHS/United States
- U01 AI037613/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- U01 AA020790/AA/NIAAA NIH HHS/United States
- R01 DA012568/DA/NIDA NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- R01 AA016893/AA/NIAAA NIH HHS/United States
- N01 CP001004/CP/NCI NIH HHS/United States
- P30 AI027767/AI/NIAID NIH HHS/United States
- U01 AI035042/AI/NIAID NIH HHS/United States
- F31 AI124794/AI/NIAID NIH HHS/United States
- P30 MH062246/MH/NIMH NIH HHS/United States
- U01 AI069434/AI/NIAID NIH HHS/United States
- U01 AI037984/AI/NIAID NIH HHS/United States
- U01 AI103397/AI/NIAID NIH HHS/United States
- R01 DA011602/DA/NIDA NIH HHS/United States
- K23 EY013707/EY/NEI NIH HHS/United States
- R01 CA165937/CA/NCI NIH HHS/United States
- U01 AI035004/AI/NIAID NIH HHS/United States
- G12 MD007583/MD/NIMHD NIH HHS/United States
- K24 DA000432/DA/NIDA NIH HHS/United States
- U01 DA036935/DA/NIDA NIH HHS/United States
- UL1 RR024131/RR/NCRR NIH HHS/United States
- U01 AI034989/AI/NIAID NIH HHS/United States
- U01 AI035041/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- UM1 AI035043/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U10 EY008057/EY/NEI NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- U01 AI034994/AI/NIAID NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- U10 EY008052/EY/NEI NIH HHS/United States
- P30 AI110527/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- K01 AI093197/AI/NIAID NIH HHS/United States
- U01 AI069918/AI/NIAID NIH HHS/United States
- K24 AI118591/AI/NIAID NIH HHS/United States
- K24 AI065298/AI/NIAID NIH HHS/United States
- U01 AI103401/AI/NIAID NIH HHS/United States
- U01 AA013566/AA/NIAAA NIH HHS/United States
- N02 CP055504/CP/NCI NIH HHS/United States
- KL2 TR002317/TR/NCATS NIH HHS/United States
- UL1 TR000083/TR/NCATS NIH HHS/United States
- P30 AI027757/AI/NIAID NIH HHS/United States
- KL2 TR000421/TR/NCATS NIH HHS/United States
- U01 AI035040/AI/NIAID NIH HHS/United States
- K01 AI131895/AI/NIAID NIH HHS/United States
- U01 AI103390/AI/NIAID NIH HHS/United States
- U01 AI034993/AI/NIAID NIH HHS/United States
- U01 AI103408/AI/NIAID NIH HHS/United States
- F31 DA037788/DA/NIDA NIH HHS/United States
- U01 AI035039/AI/NIAID NIH HHS/United States
- R01 AG053100/AG/NIA NIH HHS/United States
- U10 EY008067/EY/NEI NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- U01 HL146194/HL/NHLBI NIH HHS/United States
- U24 AA020794/AA/NIAAA NIH HHS/United States
- U01 AI068634/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- U01 HD032632/HD/NICHD NIH HHS/United States
- U01 AI042590/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

