Comparative evaluation of novel screening strategies for colorectal cancer screening in China (TARGET-C): a study protocol for a multicentre randomised controlled trial
- PMID: 31005927
- PMCID: PMC6500225
- DOI: 10.1136/bmjopen-2018-025935
Comparative evaluation of novel screening strategies for colorectal cancer screening in China (TARGET-C): a study protocol for a multicentre randomised controlled trial
Abstract
Introduction: Screening for colorectal cancer (CRC) is effective in reducing the disease burden. However, high-level evidence from randomised controlled trials on the effectiveness of CRC screening modalities is still lacking. We will conduct a large-scale multicentre randomised controlled trial in China to evaluate the effectiveness and cost-effectiveness of different CRC screening strategies.
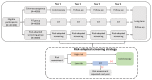
Methods and analysis: 20 000 eligible participants aged 50-74 years are enrolled in five provinces in China. After providing signed informed consent, the participants will be randomised into one of the three screening groups: (1) one-time colonoscopy (n=4000), (2) annual faecal immunochemical test (FIT) (n=8000) and (3) annual risk-adapted screening strategy (n=8000). The risk-adapted screening strategy will use an established CRC risk scoring system, the Asia-Pacific Colorectal Screening score. Participants at high risk of CRC will be referred for colonoscopy, while participants at low risk will be referred for an FIT. Information on clinical reports, epidemiological risk factors and health economic factors will be collected and stored in a web-based data management system. We will further request the participants to donate blood, faecal and saliva samples before conducting the colonoscopy. The primary outcome will be the detection rate of advanced colorectal neoplasia and the secondary outcomes will include the rates of CRC-related mortality, incidence of CRC, participation and complications. The study will last for at least 4 years and the cohort will be followed for 10 years to adequately answer the scientific questions.
Ethics and dissemination: This study was approved by the Ethics Committee of the National Cancer Center/Cancer Hospital, the Chinese Academy of Medical Sciences and Peking Union Medical College (18-013/1615). The results of the study will be submitted for publication in peer-reviewed journals and will be discussed by policy and decision makers.
Trial registration number: ChiCTR1800015506.
Keywords: advanced adenoma; colorectal cancer; early detection; randomized controlled trial; risk score.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Evaluation of a novel approach for colorectal cancer screening using an intelligent tool (INTEL-PATH CRC): a protocol for a multicentre, real-world, cohort study.BMJ Open. 2025 Jul 18;15(7):e098679. doi: 10.1136/bmjopen-2024-098679. BMJ Open. 2025. PMID: 40681202 Free PMC article.
-
A digital intake tool to avert outpatient visits in a FIT-based colorectal cancer screening population: study protocol of a multicentre, prospective non-randomized trial - the DIT-trial.BMC Gastroenterol. 2024 Jan 18;24(1):38. doi: 10.1186/s12876-023-03039-0. BMC Gastroenterol. 2024. PMID: 38238726 Free PMC article.
-
Comparative Evaluation of Participation and Diagnostic Yield of Colonoscopy vs Fecal Immunochemical Test vs Risk-Adapted Screening in Colorectal Cancer Screening: Interim Analysis of a Multicenter Randomized Controlled Trial (TARGET-C).Am J Gastroenterol. 2020 Aug;115(8):1264-1274. doi: 10.14309/ajg.0000000000000624. Am J Gastroenterol. 2020. PMID: 32282342 Clinical Trial.
-
Economic evaluations of screening strategies for the early detection of colorectal cancer in the average-risk population: A systematic literature review.PLoS One. 2019 Dec 31;14(12):e0227251. doi: 10.1371/journal.pone.0227251. eCollection 2019. PLoS One. 2019. PMID: 31891647 Free PMC article.
-
Faecal immunochemical tests versus guaiac faecal occult blood tests: what clinicians and colorectal cancer screening programme organisers need to know.Gut. 2015 Aug;64(8):1327-37. doi: 10.1136/gutjnl-2014-308074. Epub 2015 Jun 3. Gut. 2015. PMID: 26041750 Review.
Cited by
-
The relationship between public risk preference and the underuse or overuse of preventive health services in the information age.Prev Med Rep. 2024 Apr 12;41:102727. doi: 10.1016/j.pmedr.2024.102727. eCollection 2024 May. Prev Med Rep. 2024. PMID: 38633208 Free PMC article.
-
Optimising colorectal cancer screening in Shanghai, China: a modelling study.BMJ Open. 2022 May 16;12(5):e048156. doi: 10.1136/bmjopen-2020-048156. BMJ Open. 2022. PMID: 35577474 Free PMC article.
-
A scoping review of risk-stratified bowel screening: current evidence, future directions.Cancer Causes Control. 2022 May;33(5):653-685. doi: 10.1007/s10552-022-01568-9. Epub 2022 Mar 20. Cancer Causes Control. 2022. PMID: 35306592 Free PMC article.
-
Implications of Lifestyle Factors and Polygenic Risk Score for Absolute Risk Prediction of Colorectal Neoplasm and Risk-Adapted Screening.Front Mol Biosci. 2021 Jul 16;8:685410. doi: 10.3389/fmolb.2021.685410. eCollection 2021. Front Mol Biosci. 2021. PMID: 34336927 Free PMC article.
-
Validation of the Asia-Pacific colorectal screening score and its modified versions in predicting colorectal advanced neoplasia in Chinese population.BMC Cancer. 2022 Sep 7;22(1):961. doi: 10.1186/s12885-022-10047-y. BMC Cancer. 2022. PMID: 36071414 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, et al. . GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical