Protocol for the Gut Bugs Trial: a randomised double-blind placebo-controlled trial of gut microbiome transfer for the treatment of obesity in adolescents
- PMID: 31005929
- PMCID: PMC6500264
- DOI: 10.1136/bmjopen-2018-026174
Protocol for the Gut Bugs Trial: a randomised double-blind placebo-controlled trial of gut microbiome transfer for the treatment of obesity in adolescents
Abstract
Introduction: Animal studies showed that germ-free mice inoculated with normal mouse gut bacteria developed obesity, insulin resistance and higher triglyceride levels, despite similar food intake. In humans, an association has been found between obesity and gut microbiome dysbiosis. However, gut microbiome transfer has not been evaluated for the treatment of human obesity. We will examine the effectiveness of gut microbiome transfer using encapsulated material for the treatment of obesity in adolescents.
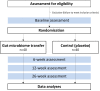
Methods and analysis: A two-arm, double-blind, placebo-controlled, randomised clinical trial of a single course of gut microbiome transfer will be conducted in 80 obese [body mass index (BMI) ≥30 kg/m2] adolescents (males and females, aged 14-18 years) in Auckland, New Zealand. Healthy lean donors (males and females, aged 18-28 years) will provide fresh stool samples from which bacteria will be isolated and double encapsulated. Participants (recipients) will be randomised at 1:1 to control (placebo) or treatment (gut microbiome transfer), stratified by sex. Recipients will receive 28 capsules over two consecutive mornings (~14 mL of frozen microbial suspension or saline). Clinical assessments will be performed at baseline, 6, 12 and 26 weeks, and will include: anthropometry, blood pressure, fasting metabolic markers, dietary intake, physical activity levels and health-related quality of life. Insulin sensitivity (Matsuda index), gut microbiota population structure characterised by 16S rRNA amplicon sequencing and body composition (using dual-energy X-ray absorptiometry) will be assessed at baseline, 6, 12 and 26 weeks. 24-hour ambulatory blood pressure monitoring will be performed at baseline and at 6 weeks. The primary outcome is BMI SD scores (SDS) at 6 weeks, with BMI SDS at 12 and 26 weeks as secondary outcomes. Other secondary outcomes include insulin sensitivity, adiposity (total body fat percentage) and gut microbial composition at 6, 12 and 26 weeks. Statistical analysis will be performed on the principle of intention to treat.
Ethics and dissemination: Ethics approval was provided by the Northern A Health and Disability Ethics Committee (Ministry of Health, New Zealand; 16/NTA/172). The trial results will be published in peer-reviewed journals and presented at international conferences.
Trial registration number: ACTRN12615001351505; Pre-results.
Keywords: adolescents; clinical trials; microbiology; obesity; paediatric endocrinology.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization. Report of the commission on ending childhood obesity. 2016. http://www.who.int/end-childhood-obesity/publications/echo-report/en/ (Accessed 29 Jan 2019).
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources