Screening and treatment to reduce severe hyperbilirubinaemia in infants in primary care (STARSHIP): a factorial stepped-wedge cluster randomised controlled trial protocol
- PMID: 31005942
- PMCID: PMC6500291
- DOI: 10.1136/bmjopen-2018-028270
Screening and treatment to reduce severe hyperbilirubinaemia in infants in primary care (STARSHIP): a factorial stepped-wedge cluster randomised controlled trial protocol
Abstract
Introduction: Jaundice caused by hyperbilirubinaemia is a physiological phenomenon in the neonatal period. However, severe hyperbilirubinaemia, when left untreated, may cause kernicterus, a severe condition resulting in lifelong neurological disabilities. Although commonly applied, visual inspection is ineffective in identifying severe hyperbilirubinaemia. We aim to investigate whether among babies cared for in primary care: (1) transcutaneous bilirubin (TcB) screening can help reduce severe hyperbilirubinaemia and (2) primary care-based (versus hospital-based) phototherapy can help reduce hospital admissions.
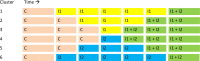
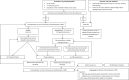
Methods and analysis: A factorial stepped-wedge cluster randomised controlled trial will be conducted in seven Dutch primary care birth centres (PCBC). Neonates born after 35 weeks of gestation and cared for at a participating PCBC for at least 2 days within the first week of life are eligible, provided they have not received phototherapy before. According to the stepped-wedge design, following a phase of 'usual care' (visual assessment and selective total serum bilirubin (TSB) quantification), either daily TcB measurement or, if indicated, phototherapy in the PCBC will be implemented (phase II). In phase III, both interventions will be evaluated in each PCBC. We aim to include 5500 neonates over 3 years.Primary outcomes are assessed at 14 days of life: (1) the proportion of neonates having experienced severe hyperbilirubinaemia (for the TcB screening intervention), defined as a TSB above the mean of the phototherapy and the exchange transfusion threshold and (2) the proportion of neonates having required hospital admission for hyperbilirubinaemia treatment (for the phototherapy intervention in primary care).
Ethics and dissemination: This study has been approved by the Medical Research Ethics Committee of the Erasmus MC Rotterdam, the Netherlands (MEC-2017-473). Written parental informed consent will be obtained. Results from this study will be published in peer-reviewed journals and presented at (inter)national meetings.
Trial registration number: NTR7187.
Keywords: neonatal jaundice; neonatal unconjugated hyperbilirubinemia; neonatology; phototherapy; primary care; transcutaneous bilirubin measurement.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study.Sci Rep. 2022 Aug 23;12(1):14385. doi: 10.1038/s41598-022-17933-2. Sci Rep. 2022. PMID: 35999237 Free PMC article. Clinical Trial.
-
Better assessment of neonatal jaundice at home (BEAT Jaundice @home): protocol for a prospective, multicentre diagnostic study.BMJ Open. 2022 Nov 17;12(11):e061897. doi: 10.1136/bmjopen-2022-061897. BMJ Open. 2022. PMID: 36396315 Free PMC article.
-
Can transcutaneous bilirubinometry safely guide phototherapy treatment of neonatal jaundice in Malawi?Paediatr Int Child Health. 2014 May;34(2):101-7. doi: 10.1179/2046905513Y.0000000050. Epub 2013 Dec 6. Paediatr Int Child Health. 2014. PMID: 24090969
-
Cochrane Review: Prophylactic phototherapy for preventing jaundice in preterm or low birth weight infants.Evid Based Child Health. 2013 Jan;8(1):204-49. doi: 10.1002/ebch.1898. Evid Based Child Health. 2013. PMID: 23878128 Review.
-
[An update on unconjugated neonatal hyperbilirubinaemia in Denmark].Ugeskr Laeger. 2020 Mar 30;182(14A):V11190671. Ugeskr Laeger. 2020. PMID: 32285792 Review. Danish.
Cited by
-
First in-human pilot study of wearable phototherapy for neonatal hyperbilirubinaemia.Eur J Pediatr. 2025 Jun 9;184(7):407. doi: 10.1007/s00431-025-06239-w. Eur J Pediatr. 2025. PMID: 40488806 Free PMC article.
-
Assessment, management, and incidence of neonatal jaundice in healthy neonates cared for in primary care: a prospective cohort study.Sci Rep. 2022 Aug 23;12(1):14385. doi: 10.1038/s41598-022-17933-2. Sci Rep. 2022. PMID: 35999237 Free PMC article. Clinical Trial.
-
Comparing Kramer's rule with transcutaneous bilirubin vs. Kramer's rule only in reducing total serum bilirubin sampling among neonates with jaundice.BMC Pediatr. 2025 Mar 6;25(1):169. doi: 10.1186/s12887-025-05423-z. BMC Pediatr. 2025. PMID: 40045227 Free PMC article.
-
Better assessment of neonatal jaundice at home (BEAT Jaundice @home): protocol for a prospective, multicentre diagnostic study.BMJ Open. 2022 Nov 17;12(11):e061897. doi: 10.1136/bmjopen-2022-061897. BMJ Open. 2022. PMID: 36396315 Free PMC article.
-
Universal screening for hyperbilirubinemia in term healthy newborns at discharge: A systematic review and meta-analysis.J Glob Health. 2022 Dec 29;12:12007. doi: 10.7189/jogh.12.12007. J Glob Health. 2022. PMID: 36579719 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
