Selective vulnerability in α-synucleinopathies
- PMID: 31006067
- PMCID: PMC6800835
- DOI: 10.1007/s00401-019-02010-2
Selective vulnerability in α-synucleinopathies
Abstract
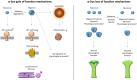
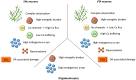
Parkinson's disease, dementia with Lewy bodies, and multiple system atrophy are neurodegenerative disorders resulting in progressive motor/cognitive deficits among other symptoms. They are characterised by stereotypical brain cell loss accompanied by the formation of proteinaceous aggregations of the protein α-synuclein (α-syn), being, therefore, termed α-synucleinopathies. Although the presence of α-syn inclusions is a common hallmark of these disorders, the exact nature of the deposited protein is specific to each disease. Different neuroanatomical regions and cellular populations manifest a differential vulnerability to the appearance of protein deposits, cell dysfunction, and cell death, leading to phenotypic diversity. The present review describes the multiple factors that contribute to the selective vulnerability in α-synucleinopathies. We explore the intrinsic cellular properties in the affected regions, including the physiological and pathophysiological roles of endogenous α-syn, the metabolic and genetic build-up of the cells and their connectivity. These factors converge with the variability of the α-syn conformational strains and their spreading capacity to dictate the phenotypic diversity and regional vulnerability of each disease. Finally, we describe the exogenous and environmental factors that potentially contribute by igniting and modulating the differential pathology in α-synucleinopathies. In conclusion, we think that it is the confluence of this disruption of the cellular metabolic state and α-syn structural equilibrium through the anatomical connectivity which appears to initiate cascades of pathological processes triggered by genetic, environmental, or stochastic events that result in the "death by a thousand cuts" profile of α-synucleinopathies.
Figures
References
-
- Acosta SA, Tajiri N, Shinozuka K, Ishikawa H, Grimmig B, Diamond DM, et al. Long-term upregulation of inflammation and suppression of cell proliferation in the brain of adult rats exposed to traumatic brain injury using the controlled cortical impact model. PLoS One. 2013;8:e53376. doi: 10.1371/journal.pone.0053376. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

