Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study
- PMID: 31006170
- PMCID: PMC6803026
- DOI: 10.1111/1471-0528.15799
Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study
Abstract
Objective: To evaluate the potential impact of intrapartum antibiotics, and their specific classes, on the infant gut microbiota in the first year of life.
Design: Prospective study of infants in the New Hampshire Birth Cohort Study (NHBCS).
Settings: Rural New Hampshire, USA.
Population or sample: Two hundred and sixty-six full-term infants from the NHBCS.
Methods: Intrapartum antibiotic use during labour and delivery was abstracted from medical records. Faecal samples collected at 6 weeks and 1 year of age were characterised by 16S rRNA sequencing, and metagenomics analysis in a subset of samples.
Exposures: Maternal exposure to antibiotics during labour and delivery.
Main outcome measure: Taxonomic and functional profiles of faecal samples.
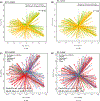
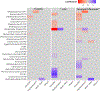
Results: Infant exposure to intrapartum antibiotics, particularly to two or more antibiotic classes, was independently associated with lower microbial diversity scores as well as a unique bacterial community at 6 weeks (GUnifrac, P = 0.02). At 1 year, infants in the penicillin-only group had significantly lower α diversity scores than infants not exposed to intrapartum antibiotics. Within the first year of life, intrapartum exposure to penicillins was related to a significantly lower increase in several taxa including Bacteroides, use of cephalosporins was associated with a significantly lower rise over time in Bifidobacterium and infants in the multi-class group experienced a significantly higher increase in Veillonella dispar.
Conclusions: Our findings suggest that intrapartum antibiotics alter the developmental trajectory of the infant gut microbiome, and specific antibiotic types may impact community composition, diversity and keystone immune training taxa.
Tweetable abstract: Class of intrapartum antibiotics administered during delivery relates to maturation of infant gut microbiota.
Keywords: Gut; infant; intestinal microbiota; intrapartum antibiotics; neonate.
© 2019 Royal College of Obstetricians and Gynaecologists.
Conflict of interest statement
Disclosure of interests
None declared. Completed disclosure of interest forms are available to view online as supporting information.
Figures

Similar articles
-
Impact of maternal intrapartum antibiotics, method of birth and breastfeeding on gut microbiota during the first year of life: a prospective cohort study.BJOG. 2016 May;123(6):983-93. doi: 10.1111/1471-0528.13601. Epub 2015 Sep 28. BJOG. 2016. PMID: 26412384
-
Impact of intrapartum antimicrobial prophylaxis upon the intestinal microbiota and the prevalence of antibiotic resistance genes in vaginally delivered full-term neonates.Microbiome. 2017 Aug 8;5(1):93. doi: 10.1186/s40168-017-0313-3. Microbiome. 2017. PMID: 28789705 Free PMC article.
-
Perinatal Antibiotic Exposure Affects the Transmission between Maternal and Neonatal Microbiota and Is Associated with Early-Onset Sepsis.mSphere. 2020 Feb 19;5(1):e00984-19. doi: 10.1128/mSphere.00984-19. mSphere. 2020. PMID: 32075882 Free PMC article.
-
Effect of intrapartum antibiotics on the intestinal microbiota of infants: a systematic review.Arch Dis Child Fetal Neonatal Ed. 2020 Mar;105(2):201-208. doi: 10.1136/archdischild-2018-316659. Epub 2019 Jul 11. Arch Dis Child Fetal Neonatal Ed. 2020. PMID: 31296695
-
The influence of prenatal and intrapartum antibiotics on intestinal microbiota colonisation in infants: A systematic review.J Infect. 2020 Aug;81(2):190-204. doi: 10.1016/j.jinf.2020.05.002. Epub 2020 May 7. J Infect. 2020. PMID: 32389786
Cited by
-
Delivery mode and perinatal antibiotics influence the predicted metabolic pathways of the gut microbiome.Sci Rep. 2021 Sep 1;11(1):17483. doi: 10.1038/s41598-021-97007-x. Sci Rep. 2021. PMID: 34471207 Free PMC article.
-
Programming Factors of Neonatal Intestinal Dysbiosis as a Cause of Disease.Int J Mol Sci. 2023 Mar 17;24(6):5723. doi: 10.3390/ijms24065723. Int J Mol Sci. 2023. PMID: 36982799 Free PMC article. Review.
-
Effects of maternal type 1 diabetes and confounding factors on neonatal microbiomes.Diabetologia. 2024 Feb;67(2):312-326. doi: 10.1007/s00125-023-06047-7. Epub 2023 Nov 29. Diabetologia. 2024. PMID: 38030736 Free PMC article.
-
Exposure to prescribed medication in early life and impacts on gut microbiota and disease development.EClinicalMedicine. 2024 Jan 23;68:102428. doi: 10.1016/j.eclinm.2024.102428. eCollection 2024 Feb. EClinicalMedicine. 2024. PMID: 38312240 Free PMC article. Review.
-
Metagenomic analysis reveals associations between salivary microbiota and body composition in early childhood.Sci Rep. 2022 Jul 29;12(1):13075. doi: 10.1038/s41598-022-14668-y. Sci Rep. 2022. PMID: 35906254 Free PMC article.
References
-
- Zeissig S, Blumberg RS. Life at the beginning: perturbation of the microbiota by antibiotics in early life and its role in health and disease. Nat Immunol 2014;15:307–10. - PubMed
-
- Sjogren YM, Tomicic S, Lundberg A, Bottcher MF, Bjorksten B, Sverremark-Ekstrom E, et al. Influence of early gut microbiota on the maturation of childhood mucosal and systemic immune responses. Clin Exp Allergy 2009;39:1842–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- NIGMS P20GM104416/NH/NIH HHS/United States
- RD83544201/U.S. Environmental Protection Agency/International
- 1P20ES018175-02/NH/NIH HHS/United States
- UH3 OD023275/OD/NIH HHS/United States
- R01 GM123014/GM/NIGMS NIH HHS/United States
- RD83459901/U.S. Environmental Protection Agency/International
- R01 LM012723/LM/NLM NIH HHS/United States
- K01 LM011985/LM/NLM NIH HHS/United States
- NIEHS P01ES022832/NH/NIH HHS/United States
- P20 ES018175/ES/NIEHS NIH HHS/United States
- NIEHS P20ES018175/NH/NIH HHS/United States
- P01 ES022832/ES/NIEHS NIH HHS/United States
- NLM K01LM011985/NH/NIH HHS/United States
- P20 GM104416/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

