Selenium supplementation suppresses immunological and serological features of lupus in B6.Sle1b mice
- PMID: 31006265
- PMCID: PMC8761364
- DOI: 10.1080/08916934.2019.1603297
Selenium supplementation suppresses immunological and serological features of lupus in B6.Sle1b mice
Abstract
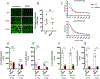
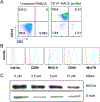
Systemic lupus erythematosus (SLE) is a debilitating multi-factorial immunological disorder characterized by increased inflammation and development of anti-nuclear autoantibodies. Selenium (Se) is an essential trace element with beneficial anti-cancer and anti-inflammatory immunological functions. In our previous proteomics study, analysis of Se-responsive markers in the circulation of Se-supplemented healthy men showed a significant increase in complement proteins. Additionally, Se supplementation prolonged the life span of lupus prone NZB/NZW-F1 mice. To better understand the protective immunological role of Se in SLE pathogenesis, we have investigated the impact of Se on B cells and macrophages using in vitro Se supplementation assays and the B6.Sle1b mouse model of lupus with an oral Se or placebo supplementation regimen. Analysis of Se-treated B6.Sle1b mice showed reduced splenomegaly and splenic cellularity compared to untreated B6. Sle1b mice. A significant reduction in total B cells and notably germinal center (GC) B cell numbers was observed. However, other cell types including T cells, Tregs, DCs and pDCs were unaffected. Consistent with reduced GC B cells there was a significant reduction in autoantibodies to dsDNA and SmRNP of the IgG2b and IgG2c subclass upon Se supplementation. We found that increased Se availability leads to impaired differentiation and maturation of macrophages from mouse bone marrow derived progenitors in vitro. Additionally, Se treatment during in vitro activation of B cells with anti-CD40L and LPS inhibited optimal B cell activation. Overall our data indicate that Se supplementation inhibits activation, differentiation and maturation of B cells and macrophages. Its specific inhibitory effect on B cell activation and GC B cell differentiation could be explored as a potential therapeutic supplement for SLE patients.
Keywords: B cells; germinal center; macrophages; selenium; systemic lupus erythematosus.
Conflict of interest statement
Declaration of Interests
The authors declare no financial conflicts of interest.
Figures




Similar articles
-
The lupus-prone NZM2410/NZW strain-derived Sle1b sublocus alters the germinal center checkpoint in female mice in a B cell-intrinsic manner.J Immunol. 2012 Dec 15;189(12):5667-81. doi: 10.4049/jimmunol.1201661. Epub 2012 Nov 9. J Immunol. 2012. PMID: 23144494 Free PMC article.
-
Antigen-specific responses and ANA production in B6.Sle1b mice: a role for SAP.J Autoimmun. 2008 Dec;31(4):345-53. doi: 10.1016/j.jaut.2008.08.002. Epub 2008 Oct 8. J Autoimmun. 2008. PMID: 18845419 Free PMC article.
-
IL-21 has a pathogenic role in a lupus-prone mouse model and its blockade with IL-21R.Fc reduces disease progression.J Immunol. 2007 Mar 15;178(6):3822-30. doi: 10.4049/jimmunol.178.6.3822. J Immunol. 2007. PMID: 17339481
-
IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity.J Exp Med. 2016 May 2;213(5):715-32. doi: 10.1084/jem.20151722. Epub 2016 Apr 11. J Exp Med. 2016. PMID: 27069112 Free PMC article.
-
B-cell-tropic interleukins in murine systemic lupus erythematosus (SLE) 1.Immunol Rev. 1984 Apr;78:159-83. doi: 10.1111/j.1600-065x.1984.tb00481.x. Immunol Rev. 1984. PMID: 6429033 Review.
Cited by
-
Selenium Modulates the Allergic Response to Whey Protein in a Mouse Model for Cow's Milk Allergy.Nutrients. 2021 Jul 22;13(8):2479. doi: 10.3390/nu13082479. Nutrients. 2021. PMID: 34444651 Free PMC article.
-
Selenization of S. cerevisiae increases its protective potential in experimental autoimmune encephalomyelitis by triggering an intestinal immunomodulatory loop.Sci Rep. 2020 Dec 17;10(1):22190. doi: 10.1038/s41598-020-79102-7. Sci Rep. 2020. PMID: 33335128 Free PMC article.
-
Short-Term and Long-Term Effects of Electronic Cigarettes on Mouse Lungs Following Nose-Only Exposures.Chem Res Toxicol. 2025 Jun 16;38(6):1019-1036. doi: 10.1021/acs.chemrestox.4c00525. Epub 2025 May 22. Chem Res Toxicol. 2025. PMID: 40401807
-
Immunomodulatory Effects of Diet and Nutrients in Systemic Lupus Erythematosus (SLE): A Systematic Review.Front Immunol. 2020 Jul 22;11:1477. doi: 10.3389/fimmu.2020.01477. eCollection 2020. Front Immunol. 2020. PMID: 32793202 Free PMC article.
-
Nutritional Approaches to Modulate Cardiovascular Disease Risk in Systemic Lupus Erythematosus: A Literature Review.Nutrients. 2023 Feb 19;15(4):1036. doi: 10.3390/nu15041036. Nutrients. 2023. PMID: 36839394 Free PMC article. Review.
References
-
- Kaul A, Gordon C, Crow MK, et al. Systemic lupus erythematosus. Nat Rev Dis Primers 2016; 2: 16039. - PubMed
-
- Pisetsky DS. Anti-DNA antibodies--quintessential biomarkers of SLE. Nat Rev Rheumatol 2016; 12(2): 102–10. - PubMed
-
- Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity 2008; 28(1): 18–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
