Activation of ADAM17 (A Disintegrin and Metalloprotease 17) on Glutamatergic Neurons Selectively Promotes Sympathoexcitation
- PMID: 31006330
- PMCID: PMC6506373
- DOI: 10.1161/HYPERTENSIONAHA.119.12832
Activation of ADAM17 (A Disintegrin and Metalloprotease 17) on Glutamatergic Neurons Selectively Promotes Sympathoexcitation
Abstract
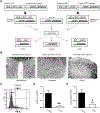
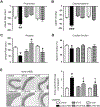
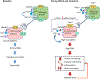
Chronic activation of the brain renin-angiotensin system contributes to the development of hypertension by altering autonomic balance. Beyond the essential role of Ang II (angiotensin II) type 1 receptors, ADAM17 (A disintegrin and metalloprotease 17) is also found to promote brain renin-angiotensin system overactivation. ADAM17 is robustly expressed in various cell types within the central nervous system. The aim of this study was to determine whether ADAM17 modulates presympathetic neuronal activity to promote autonomic dysregulation in salt-sensitive hypertension. To test our hypothesis, ADAM17 was selectively knocked down in glutamatergic neurons using Cre-loxP technology. In mice lacking ADAM17 in glutamatergic neurons, the blood pressure increase induced by deoxycorticosterone acetate-salt treatment was blunted. Deoxycorticosterone acetate-salt significantly elevated cardiac and vascular sympathetic drive in control mice, while such effects were reduced in mice with ADAM17 knockdown. This blunted sympathoexcitation was extended to the spleen, with a lesser activation of the peripheral immune system, translating into a sequestration of circulating T cells within this organ, compared with controls. Within the paraventricular nucleus, Ang II-induced activation of kidney-related presympathetic glutamatergic neurons was reduced in ADAM17 knockdown mice, with the majority of cells no longer responding to Ang II stimulation, confirming the supportive role of ADAM17 in increasing presympathetic neuronal activity. Overall, our data highlight the pivotal role of neuronal ADAM17 in regulating sympathetic activity and demonstrate that activation of ADAM17 in glutamatergic neurons leads to a selective increase of sympathetic output, but not vagal tone, to specific organs, ultimately contributing to dysautonomia and salt-sensitive hypertension.
Keywords: central nervous system; immune system; mice; neurons; renin-angiotensin system.
Conflict of interest statement
Conflict of interest/Disclosure:
None.
Figures



References
-
- Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

