A Common Neuroendocrine Substrate for Diverse General Anesthetics and Sleep
- PMID: 31006556
- PMCID: PMC6554048
- DOI: 10.1016/j.neuron.2019.03.033
A Common Neuroendocrine Substrate for Diverse General Anesthetics and Sleep
Abstract
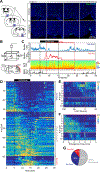
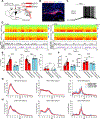
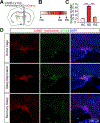
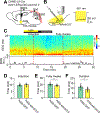
How general anesthesia (GA) induces loss of consciousness remains unclear, and whether diverse anesthetic drugs and sleep share a common neural pathway is unknown. Previous studies have revealed that many GA drugs inhibit neural activity through targeting GABA receptors. Here, using Fos staining, ex vivo brain slice recording, and in vivo multi-channel electrophysiology, we discovered a core ensemble of hypothalamic neurons in and near the supraoptic nucleus, consisting primarily of neuroendocrine cells, which are persistently and commonly activated by multiple classes of GA drugs. Remarkably, chemogenetic or brief optogenetic activations of these anesthesia-activated neurons (AANs) strongly promote slow-wave sleep and potentiates GA, whereas conditional ablation or inhibition of AANs led to diminished slow-wave oscillation, significant loss of sleep, and shortened durations of GA. These findings identify a common neural substrate underlying diverse GA drugs and natural sleep and reveal a crucial role of the neuroendocrine system in regulating global brain states. VIDEO ABSTRACT.
Keywords: activity-dependent labeling; general anesthesia; neuroendocrine cells; sleep.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures




References
-
- Bellavance MA, Takatoh J, Lu J, Demers M, Kleinfeld D, Wang F, and Deschenes M (2017). Parallel Inhibitory and Excitatory Trigemino-Facial Feedback Circuitry for Reflexive Vibrissa Movement. Neuron 95, 722–723. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

