Lineage Tracing Reveals a Subset of Reserve Muscle Stem Cells Capable of Clonal Expansion under Stress
- PMID: 31006621
- PMCID: PMC6597014
- DOI: 10.1016/j.stem.2019.03.020
Lineage Tracing Reveals a Subset of Reserve Muscle Stem Cells Capable of Clonal Expansion under Stress
Abstract
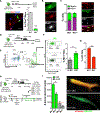
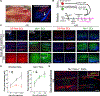
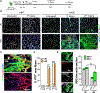
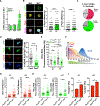
Stem cell heterogeneity is recognized as functionally relevant for tissue homeostasis and repair. The identity, context dependence, and regulation of skeletal muscle satellite cell (SC) subsets remains poorly understood. We identify a minor subset of Pax7+ SCs that is indelibly marked by an inducible Mx1-Cre transgene in vivo, is enriched for Pax3 expression, and has reduced ROS (reactive oxygen species) levels. Mx1+ SCs possess potent stem cell activity upon transplantation but minimally contribute to endogenous muscle repair, due to their relative low abundance. In contrast, a dramatic clonal expansion of Mx1+ SCs allows extensive contribution to muscle repair and niche repopulation upon selective pressure of radiation stress, consistent with reserve stem cell (RSC) properties. Loss of Pax3 in RSCs increased ROS content and diminished survival and stress tolerance. These observations demonstrate that the Pax7+ SC pool contains a discrete population of radiotolerant RSCs that undergo clonal expansion under severe stress.
Keywords: Mx1; Pax3; ROS; clonal expansion; muscle stem cells; radiation; reserve stem cell; satellite cells; stress; tissue regeneration.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures



References
-
- Blanpain C, Mohrin M, Sotiropoulou PA, and Passegué E (2011). DNA-damage response in tissue-specific and cancer stem cells. Cell Stem Cell 8, 16–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

