PAX3 Confers Functional Heterogeneity in Skeletal Muscle Stem Cell Responses to Environmental Stress
- PMID: 31006622
- PMCID: PMC6628901
- DOI: 10.1016/j.stem.2019.03.019
PAX3 Confers Functional Heterogeneity in Skeletal Muscle Stem Cell Responses to Environmental Stress
Abstract
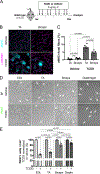
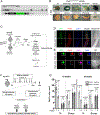
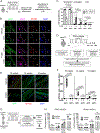
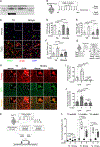
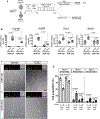
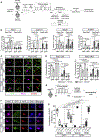
Muscle satellite cells (MuSCs) are the quiescent muscle stem cells required for adult skeletal muscle repair. The impact of environmental stress such as pollution on MuSC behavior remains unexplored. We evaluated the impact of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) exposure, a ubiquitous and highly toxic pollutant, on MuSCs by combining in vivo mouse molecular genetic models with ex vivo studies. While all MuSCs express the transcription factor PAX7, we show that a subset also express PAX3 and exhibit resistance to environmental stress. Upon systemic TCDD treatment, PAX3-negative MuSCs display impaired survival, atypical activation, and sporadic differentiation through xenobiotic aryl hydrocarbon receptor signaling. We further show that PAX3-positive MuSCs become sensitized to environmental stress when PAX3 function is impaired and that PAX3-mediated induction of mTORC1 is required for protection. Our study, therefore, identifies a functional heterogeneity of MuSCs in response to environmental stress controlled by PAX3.
Keywords: AHR; G(alert); PAX3; TCDD; environmental stress; muscle stem cells; satellite cells; skeletal muscle.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF INTERESTS
The authors declare no competing interests.
Figures

References
-
- Bismuth K, and Relaix F (2010). Genetic regulation of skeletal muscle development. Exp Cell Res 316, 3081–3086. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

