Increased luminescence of the GloSensor cAMP assay in LβT2 cells does not correlate with cAMP accumulation under low pH conditions
- PMID: 31006726
- PMCID: PMC6708853
- DOI: 10.1262/jrd.2018-153
Increased luminescence of the GloSensor cAMP assay in LβT2 cells does not correlate with cAMP accumulation under low pH conditions
Abstract
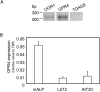
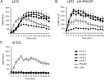
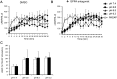
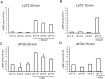
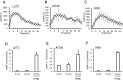
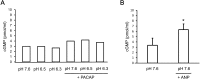
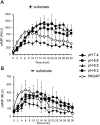
Cyclic adenosine monophosphate (cAMP) plays a pivotal role in gonadotrope responses in the pituitary. Gonadotropin-releasing hormone (GnRH) mediated synthesis and secretion of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) are regulated by both the Gs/cAMP and Gq/Ca2+ signaling pathways. Pituitary adenylate cyclase-activating polypeptide (PACAP) also regulates GnRH responsiveness in gonadotropes through the PACAP receptor, which activates the Gs/cAMP signaling pathway. Therefore, measuring intracellular cAMP levels is important for elucidating the molecular mechanisms of FSH and LH synthesis and secretion in gonadotropes. The GloSensor cAMP assay is useful for detecting cAMP levels in intact, living cells. In this study, we found that increased GloSensor luminescence intensity did not correlate with cAMP accumulation in LβT2 cells under low pH conditions. This result indicates that cell type and condition must be considered when using GloSensor cAMP.
Keywords: Cyclic adenosine monophosphate (cAMP); GloSensor; Low pH; Luminescence; LβT2.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

