Antenatal Multiple Micronutrient Supplementation Compared to Iron-Folic Acid Affects Micronutrient Status but Does Not Eliminate Deficiencies in a Randomized Controlled Trial Among Pregnant Women of Rural Bangladesh
- PMID: 31006806
- PMCID: PMC6602890
- DOI: 10.1093/jn/nxz046
Antenatal Multiple Micronutrient Supplementation Compared to Iron-Folic Acid Affects Micronutrient Status but Does Not Eliminate Deficiencies in a Randomized Controlled Trial Among Pregnant Women of Rural Bangladesh
Abstract
Background: Antenatal multiple micronutrient (MM) supplementation improves birth outcomes relative to iron-folic acid (IFA) in developing countries, but limited data exist on its impact on pregnancy micronutrient status.
Objective: We assessed the efficacy of a daily MM (15 nutrients) compared with IFA supplement, each providing approximately 1 RDA of nutrients and given beginning at pregnancy ascertainment, on late pregnancy micronutrient status of women in rural Bangladesh. Secondarily, we explored other contributors to pregnancy micronutrient status.
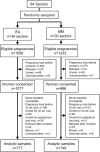
Methods: Within a double-masked trial (JiVitA-3) among 44,500 pregnant women, micronutrient status indicators were assessed in n = 1526 women, allocated by cluster to receive daily MM (n = 749) or IFA (n = 777), at 10 wk (baseline: before supplementation) and 32 wk (during supplementation) gestation. Efficacy of MM supplementation on micronutrient status indicators at 32 wk was assessed, controlling for baseline status and other covariates (e.g., inflammation and season), in regression models.
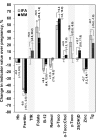
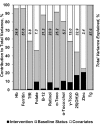
Results: Baseline status was comparable by intervention. Prevalence of deficiency among all participants was as follows: anemia, 20.6%; iron by ferritin, 4.0%; iron by transferrin receptor, 4.7%; folate, 2.5%; vitamin B-12, 35.4%; vitamin A, 6.7%; vitamin E, 57.7%; vitamin D, 64.0%; zinc, 13.4%; and iodine, 2.6%. At 32 wk gestation, vitamin B-12, A, and D and zinc status indicators were 3.7-13.7% higher, and ferritin, γ-tocopherol, and thyroglobulin indicators were 8.7-16.6% lower, for the MM group compared with the IFA group, with a 15-38% lower prevalence of deficiencies of vitamins B-12, A, and D and zinc (all P < 0.05). However, indicators typically suggested worsening status during pregnancy, even with supplementation, and baseline status or other covariates were more strongly associated with late pregnancy indicators than was MM supplementation.
Conclusions: Rural Bangladeshi women commonly entered pregnancy deficient in micronutrients other than iron and folic acid. Supplementation with MM improved micronutrient status, although deficiencies persisted. Preconception supplementation or higher nutrient doses may be warranted to support nutritional demands of pregnancy in undernourished populations. This trial was registered at clinicaltrials.gov as NCT00860470.
Keywords: Bangladesh; South Asia; antenatal; micronutrients; minerals; pregnancy; supplementation; trial; vitamins.
Copyright © American Society for Nutrition 2019.
Figures
Similar articles
-
Newborn micronutrient status biomarkers in a cluster-randomized trial of antenatal multiple micronutrient compared with iron folic acid supplementation in rural Bangladesh.Am J Clin Nutr. 2020 Nov 11;112(5):1328-1337. doi: 10.1093/ajcn/nqaa223. Am J Clin Nutr. 2020. PMID: 32844185 Free PMC article. Clinical Trial.
-
Antenatal supplementation with micronutrients and biochemical indicators of status and subclinical infection in rural Nepal.Am J Clin Nutr. 2006 Apr;83(4):788-94. doi: 10.1093/ajcn/83.4.788. Am J Clin Nutr. 2006. PMID: 16600929 Clinical Trial.
-
Effect of maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial.JAMA. 2014 Dec 24-31;312(24):2649-58. doi: 10.1001/jama.2014.16819. JAMA. 2014. PMID: 25536256 Clinical Trial.
-
Prevalence of multiple micronutrient deficiencies amongst pregnant women in a rural area of Haryana.Indian J Pediatr. 2004 Nov;71(11):1007-14. doi: 10.1007/BF02828117. Indian J Pediatr. 2004. PMID: 15572822 Review.
-
Vitamin and Mineral Supplementation During Pregnancy on Maternal, Birth, Child Health and Development Outcomes in Low- and Middle-Income Countries: A Systematic Review and Meta-Analysis.Nutrients. 2020 Feb 14;12(2):491. doi: 10.3390/nu12020491. Nutrients. 2020. PMID: 32075071 Free PMC article.
Cited by
-
Micronutrient Supplementation for Pregnant and Lactating Women to Improve Maternal and Infant Nutritional Status in Low- and Middle-Income Countries: Protocol for a Systematic Review and Meta-analysis.JMIR Res Protoc. 2022 Aug 30;11(8):e40134. doi: 10.2196/40134. JMIR Res Protoc. 2022. PMID: 36040761 Free PMC article.
-
Nutrient density of Bangladeshi foods and its application in planning diet for pregnant women.PLoS One. 2024 Jan 17;19(1):e0296831. doi: 10.1371/journal.pone.0296831. eCollection 2024. PLoS One. 2024. PMID: 38232085 Free PMC article.
-
Prenatal supplementation with multiple micronutrient supplements or medium-quantity lipid-based nutrient supplements has limited effects on child growth up to 24 months in rural Niger: a secondary analysis of a cluster randomized trial.Am J Clin Nutr. 2022 Mar 4;115(3):738-748. doi: 10.1093/ajcn/nqab404. Am J Clin Nutr. 2022. PMID: 34871344 Free PMC article. Clinical Trial.
-
Small-Quantity Lipid-Based Nutrient Supplements Do Not Affect Plasma or Milk Retinol Concentrations Among Malawian Mothers, or Plasma Retinol Concentrations among Young Malawian or Ghanaian Children in Two Randomized Trials.J Nutr. 2021 Apr 8;151(4):1029-1037. doi: 10.1093/jn/nxaa439. J Nutr. 2021. PMID: 33561214 Free PMC article. Clinical Trial.
-
Fortified Balanced Energy-Protein Supplementation, Maternal Anemia, and Gestational Weight Gain: A Randomized Controlled Efficacy Trial among Pregnant Women in Rural Burkina Faso.J Nutr. 2022 Oct 6;152(10):2277-2286. doi: 10.1093/jn/nxac171. J Nutr. 2022. PMID: 35906874 Free PMC article. Clinical Trial.
References
-
- West KP Jr, Shamim AA, Mehra S, Labrique AB, Ali H, Shaikh S, Klemm RD, Wu LS, Mitra M, Haque R et al. .. Effect of maternal multiple micronutrient vs iron–folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial. JAMA. 2014;312:2649–58. - PubMed
-
- UNICEF, World Health Organization, and United Nations University. Composition of a multi-micronutrient supplement to be used in pilot programmes among pregnant women in developing countries: report of a United Nations Children's Fund (UNICEF), World Health Organization (WHO) and United Nations University Workshop. Geneva (Switzerland): World Health Organization; 1999.

