Free-breathing cine imaging with motion-corrected reconstruction at 3T using SPiral Acquisition with Respiratory correction and Cardiac Self-gating (SPARCS)
- PMID: 31006916
- PMCID: PMC6510595
- DOI: 10.1002/mrm.27763
Free-breathing cine imaging with motion-corrected reconstruction at 3T using SPiral Acquisition with Respiratory correction and Cardiac Self-gating (SPARCS)
Abstract
Purpose: To develop a continuous-acquisition cardiac self-gated spiral pulse sequence and a respiratory motion-compensated reconstruction strategy for free-breathing cine imaging.
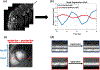
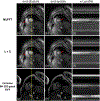
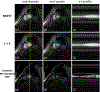
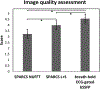
Methods: Cine data were acquired continuously on a 3T scanner for 8 seconds per slice without ECG gating or breath-holding, using a golden-angle gradient echo spiral pulse sequence. Cardiac motion information was extracted by applying principal component analysis on the gridded 8 × 8 k-space center data. Respiratory motion was corrected by rigid registration on each heartbeat. Images were reconstructed using a low-rank and sparse (L+S) technique. This strategy was evaluated in 37 healthy subjects and 8 subjects undergoing clinical cardiac MR studies. Image quality was scored (1-5 scale) in a blinded fashion by 2 experienced cardiologists. In 13 subjects with whole-heart coverage, left ventricular ejection fraction (LVEF) from SPiral Acquisition with Respiratory correction and Cardiac Self-gating (SPARCS) was compared to that from a standard ECG-gated breath-hold balanced steady-state free precession (bSSFP) cine sequence.
Results: The self-gated signal was successfully extracted in all cases and demonstrated close agreement with the acquired ECG signal (mean bias, -0.22 ms). The mean image score across all subjects was 4.0 for reconstruction using the L+S model. There was good agreement between the LVEF derived from SPARCS and the gold-standard bSSFP technique.
Conclusion: SPARCS successfully images cardiac function without the need for ECG gating or breath-holding. With an 8-second data acquisition per slice, whole-heart cine images with clinically acceptable spatial and temporal resolution and image quality can be acquired in <90 seconds of free-breathing acquisition.
Keywords: cardiac MRI; golden angle; low-rank and sparse; motion correction; self-gating; spiral trajectory.
© 2019 International Society for Magnetic Resonance in Medicine.
Figures


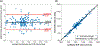



Similar articles
-
Dual-excitation flip-angle simultaneous cine and T1 mapping using spiral acquisition with respiratory and cardiac self-gating.Magn Reson Med. 2021 Jul;86(1):82-96. doi: 10.1002/mrm.28675. Epub 2021 Feb 15. Magn Reson Med. 2021. PMID: 33590591 Free PMC article.
-
3D self-gated cardiac cine imaging at 3 Tesla using stack-of-stars bSSFP with tiny golden angles and compressed sensing.Magn Reson Med. 2019 May;81(5):3234-3244. doi: 10.1002/mrm.27612. Epub 2018 Nov 25. Magn Reson Med. 2019. PMID: 30474151
-
Manifold learning based ECG-free free-breathing cardiac CINE MRI.J Magn Reson Imaging. 2015 Jun;41(6):1521-7. doi: 10.1002/jmri.24731. Epub 2014 Aug 14. J Magn Reson Imaging. 2015. PMID: 25124545
-
Cardiovascular magnetic resonance physics for clinicians: part I.J Cardiovasc Magn Reson. 2010 Nov 30;12(1):71. doi: 10.1186/1532-429X-12-71. J Cardiovasc Magn Reson. 2010. PMID: 21118531 Free PMC article. Review.
-
High efficiency free-breathing 3D thoracic aorta vessel wall imaging using self-gating image reconstruction.Magn Reson Imaging. 2024 Apr;107:80-87. doi: 10.1016/j.mri.2024.01.009. Epub 2024 Jan 17. Magn Reson Imaging. 2024. PMID: 38237694 Review.
Cited by
-
Free-Breathing and Ungated Dynamic MRI Using Navigator-Less Spiral SToRM.IEEE Trans Med Imaging. 2020 Dec;39(12):3933-3943. doi: 10.1109/TMI.2020.3008329. Epub 2020 Nov 30. IEEE Trans Med Imaging. 2020. PMID: 32746136 Free PMC article.
-
Improved visualization of free-running cardiac magnetic resonance by respiratory phase using principal component analysis.Res Diagn Interv Imaging. 2023 Oct 3;8:100035. doi: 10.1016/j.redii.2023.100035. eCollection 2023 Dec. Res Diagn Interv Imaging. 2023. PMID: 39678163 Free PMC article.
-
Heart Rate-Independent 3D Myocardial Blood Oxygen Level-Dependent MRI at 3.0 T with Simultaneous 13N-Ammonia PET Validation.Radiology. 2020 Apr;295(1):82-93. doi: 10.1148/radiol.2020191456. Epub 2020 Feb 25. Radiology. 2020. PMID: 32096705 Free PMC article.
-
3D joint T1/T1 ρ/T2 mapping and water-fat imaging for contrast-agent free myocardial tissue characterization at 1.5T.Magn Reson Med. 2025 Jun;93(6):2297-2310. doi: 10.1002/mrm.30397. Epub 2025 Feb 21. Magn Reson Med. 2025. PMID: 39981990 Free PMC article.
-
Dual-excitation flip-angle simultaneous cine and T1 mapping using spiral acquisition with respiratory and cardiac self-gating.Magn Reson Med. 2021 Jul;86(1):82-96. doi: 10.1002/mrm.28675. Epub 2021 Feb 15. Magn Reson Med. 2021. PMID: 33590591 Free PMC article.
References
-
- Shetty AN. Suppression of Radiofrequency Interference in Cardiac Gated MRI: A Simple Design. Magn. Reson. Med 1988;8:84–88. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

