Tuning the In Vivo Transport of Anticancer Drugs Using Renal-Clearable Gold Nanoparticles
- PMID: 31006932
- PMCID: PMC6555664
- DOI: 10.1002/anie.201903256
Tuning the In Vivo Transport of Anticancer Drugs Using Renal-Clearable Gold Nanoparticles
Abstract
Precise control of in vivo transport of anticancer drugs in normal and cancerous tissues with engineered nanoparticles is key to the future success of cancer nanomedicines in clinics. This requires a fundamental understanding of how engineered nanoparticles impact the targeting-clearance and permeation-retention paradoxes in the anticancer-drug delivery. Herein, we systematically investigated how renal-clearable gold nanoparticles (AuNPs) affect the permeation, distribution, and retention of the anticancer drug doxorubicin in both cancerous and normal tissues. Renal-clearable AuNPs retain the advantages of the free drug, including rapid tumor targeting and high tumor vascular permeability. The renal-clearable AuNPs also accelerated body clearance of off-target drug via renal elimination. These results clearly indicate that diverse in vivo transport behaviors of engineered nanoparticles can be used to reconcile long-standing paradoxes in the anticancer drug delivery.
Keywords: drug delivery; enhanced permeability and retention effect; gold nanoparticles; renal clearance; tumor targeting.
© 2019 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
The authors declare no conflict of interests.
Figures

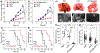


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

