Excitation parameters optimized for coherent anti-Stokes Raman scattering imaging of myelinated tissue
- PMID: 31007003
- PMCID: PMC6990057
- DOI: 10.1117/1.JBO.24.4.046502
Excitation parameters optimized for coherent anti-Stokes Raman scattering imaging of myelinated tissue
Abstract
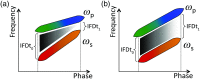
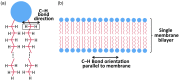
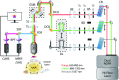
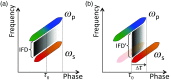
Coherent anti-Stokes Raman scattering (CARS) generates a strong label-free signal in the long wavenumber C─H stretching region. Lipid-rich myelinated tissues, such as brain and spinal cord, would appear to be ideal subjects for imaging with CARS laser-scanning microscopy. However, the highly ordered, biochemically complex, and highly scattering nature of such tissues complicate the use of the technique. A CARS microscopy approach is presented that overcomes the challenges of imaging myelinated tissue to achieve chemically and orientationally sensitive high-resolution images.
Keywords: Raman scattering; coherent anti-Stokes Raman scattering; dispersion; myelin; nonlinear optics; polarization.
Figures



Similar articles
-
Quantitative myelin imaging with coherent anti-Stokes Raman scattering microscopy: alleviating the excitation polarization dependence with circularly polarized laser beams.Opt Express. 2009 Oct 12;17(21):18419-32. doi: 10.1364/OE.17.018419. Opt Express. 2009. PMID: 20372572
-
In vivo coherent anti-Stokes Raman scattering imaging of sciatic nerve tissue.J Microsc. 2007 Feb;225(Pt 2):175-82. doi: 10.1111/j.1365-2818.2007.01729.x. J Microsc. 2007. PMID: 17359252 Free PMC article.
-
Ex vivo and in vivo imaging of myelin fibers in mouse brain by coherent anti-Stokes Raman scattering microscopy.Opt Express. 2008 Nov 24;16(24):19396-409. doi: 10.1364/oe.16.019396. Opt Express. 2008. PMID: 19030027 Free PMC article.
-
Label-free imaging of lipid dynamics using Coherent Anti-stokes Raman Scattering (CARS) and Stimulated Raman Scattering (SRS) microscopy.Curr Opin Genet Dev. 2011 Oct;21(5):585-90. doi: 10.1016/j.gde.2011.09.003. Epub 2011 Sep 22. Curr Opin Genet Dev. 2011. PMID: 21945002 Free PMC article. Review.
-
Shedding new light on lipid biology with coherent anti-Stokes Raman scattering microscopy.J Lipid Res. 2010 Nov;51(11):3091-102. doi: 10.1194/jlr.R008730. Epub 2010 Aug 16. J Lipid Res. 2010. PMID: 20713649 Free PMC article. Review.
Cited by
-
Spectral focusing-based stimulated Raman scattering microscopy using compact glass blocks for adjustable dispersion.Biomed Opt Express. 2023 May 4;14(6):2510-2522. doi: 10.1364/BOE.486753. eCollection 2023 Jun 1. Biomed Opt Express. 2023. PMID: 37342685 Free PMC article.
-
Vibrational spectroscopy and multiphoton microscopy for label-free visualization of nervous system degeneration and regeneration.Biophys Rev. 2023 Oct 5;16(2):219-235. doi: 10.1007/s12551-023-01158-2. eCollection 2024 Apr. Biophys Rev. 2023. PMID: 38737209 Free PMC article. Review.
-
Coherent Raman scattering microscopy: capable solution in search of a larger audience.J Biomed Opt. 2021 Jun;26(6):060601. doi: 10.1117/1.JBO.26.6.060601. J Biomed Opt. 2021. PMID: 34085436 Free PMC article.
-
Advances in nonlinear optical microscopy techniques for in vivo and in vitro neuroimaging.Biophys Rev. 2021 Aug 31;13(6):1199-1217. doi: 10.1007/s12551-021-00832-7. eCollection 2021 Dec. Biophys Rev. 2021. PMID: 35047093 Free PMC article. Review.
References
-
- Eckhardt G., Bortfeld D. P., Geller M., “Stimulated emission of Stokes and anti‐Stokes Raman lines from diamond, calcite, and α‐sulfur single crystals,” Appl. Phys. Lett. 3(8), 137–138 (1963).APPLAB10.1063/1.1753903 - DOI
-
- Tolles W. M., et al. , “A review of the theory and application of coherent anti-Stokes Raman spectroscopy (CARS),” Appl. Spectrosc. 31(4), 253–271 (2016).APSPA410.1366/000370277774463625 - DOI

