Establishing an electronic health record-supported approach for outreach to and recruitment of persons at high risk of type 2 diabetes in clinical trials: The vitamin D and type 2 diabetes (D2d) study experience
- PMID: 31007049
- PMCID: PMC6764596
- DOI: 10.1177/1740774519839062
Establishing an electronic health record-supported approach for outreach to and recruitment of persons at high risk of type 2 diabetes in clinical trials: The vitamin D and type 2 diabetes (D2d) study experience
Abstract
Aims: To establish recruitment approaches that leverage electronic health records in multicenter prediabetes/diabetes clinical trials and compare recruitment outcomes between electronic health record-supported and conventional recruitment methods.
Methods: Observational analysis of recruitment approaches in the vitamin D and type 2 diabetes (D2d) study, a multicenter trial in participants with prediabetes. Outcomes were adoption of electronic health record-supported recruitment approaches by sites, number of participants screened, recruitment performance (proportion screened who were randomized), and characteristics of participants from electronic health record-supported versus non-electronic health record methods.
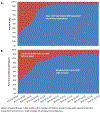
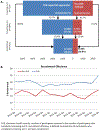
Results: In total, 2423 participants were randomized: 1920 from electronic health record (mean age of 60 years, 41% women, 68% White) and 503 from non-electronic health record sources (mean age of 56.9 years, 58% women, 61% White). Electronic health record-supported recruitment was adopted by 21 of 22 sites. Electronic health record-supported recruitment was associated with more participants screened versus non-electronic health record methods (4969 vs 2166 participants screened), higher performance (38.6% vs 22.7%), and more randomizations (1918 vs 505). Participants recruited via electronic health record were older, included fewer women and minorities, and reported higher use of dietary supplements. Electronic health record-supported recruitment was incorporated in diverse clinical environments, engaging clinicians either at the individual or the healthcare system level.
Conclusion: Establishing electronic health record-supported recruitment approaches across a multicenter prediabetes/diabetes trial is feasible and can be adopted by diverse clinical environments.
Trial registration: ClinicalTrials.gov NCT01942694.
Keywords: Prediabetes; diabetes; health records; recruitment; trial.
Conflict of interest statement
Conflict of Interest
The Author(s) declare(s) that there is no conflict of interest.
Figures
References
-
- Cook WA and Doorenbos AZ. Indications of Recruitment Challenges in Research with U.S. Military Service Members: A ClinicalTrials.gov Review. Mil Med 2017; 182: e1580–e1587. - PMC - PubMed
-
- Weisfeld VD, English RA, Claiborne AB, et al. Public engagement and clinical trials : new models and disruptive technologies : workshop summary. Washington, D.C.: National Academies Press, 2012, p.xvi, 124 p. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

