Strategies for Overcoming Barriers to Adopting Biosimilars and Achieving Goals of the Biologics Price Competition and Innovation Act: A Survey of Managed Care and Specialty Pharmacy Professionals
- PMID: 31007119
- PMCID: PMC10397695
- DOI: 10.18553/jmcp.2019.18412
Strategies for Overcoming Barriers to Adopting Biosimilars and Achieving Goals of the Biologics Price Competition and Innovation Act: A Survey of Managed Care and Specialty Pharmacy Professionals
Abstract
Background: The Biologics Price Competition and Innovation Act (BPCIA) of 2009, which included pathways for FDA approval of biosimilar products, was designed to promote more affordable, expanded patient access to biologic therapies. Achieving these BPCIA goals depends on overcoming formidable barriers to biosimilar adoption. Managed care and specialty pharmacy professionals are uniquely qualified to inform initiatives to address these barriers.
Objective: To assess perceptions regarding strategies for overcoming barriers to biosimilar adoption among managed care and specialty pharmacy professionals by conducting a survey study.
Methods: Invitations to complete the online survey were emailed by the Academy of Managed Care Pharmacy (AMCP) to members and customers and to contacts sourced from a commercial database. In addition to questions on respondent demographics and perceptions of biosimilars, the survey listed 16 strategies for overcoming key barriers to biosimilar adoption. On a 5-point scale, participants rated their opinion on the likelihood that each strategy would have the potential to assist in achieving BPCIA goals. The survey also listed 6 barriers to biosimilar adoption. On a 5-point scale, participants rated their perceived difficulty in overcoming each barrier. The survey concluded with an open-text item that asked participants to list 3 additional strategies for overcoming biosimilar adoption barriers. Response frequencies were calculated to describe participants' ratings of the strategies and barriers. Statistical analyses were conducted to assess whether the ratings differed among respondents grouped by work organization. For the open-text item, we conducted qualitative content analyses to categorize strategies by stakeholder groups that might take primary implementation roles.
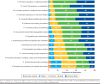
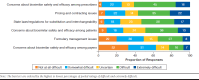
Results: A total of 300 managed care and specialty pharmacy professionals completed the survey. There was considerable variation in the preferences, policies, and practices regarding biosimilar adoption among respondents' work organizations. Responses to several survey items reflected positive attitudes about the safety and efficacy of biosimilars; for example, 84% agreed or strongly agreed that FDA-approved biosimilars are safe and effective for patients who switch from a reference biologic. Based on pooled percentages for ratings of likely and extremely likely to overcome barriers to biosimilar adoption, the highest-rated strategies were for prescriber education about evidence from switching studies (91%) and FDA guidance on pharmacy-level substitution of reference biologics with biosimilars (90%). The lowest-rated strategies were for requiring therapeutic drug monitoring for patients who switch to biosimilars (39%) and using quotas to incentivize providers to prescribe biosimilars (40%). For the qualitative analysis, the highest numbers of respondents' suggested strategies indicated primary implementation roles of biosimilar manufacturers (40%), the federal government (26%), and managed care organizations (15%).
Conclusions: Reflecting the unique knowledge, perspectives, and practices of managed care and specialty pharmacy professionals, the study findings are relevant to informing and advancing initiatives for achieving BPCIA goals.
Disclosures: The survey study reported in this article was part of a continuing education program funded by an independent educational grant, which was awarded by Sandoz, a Novartis Division, to PRIME Education. The Academy of Managed Care Pharmacy (AMCP) received grant funding from PRIME to assist in developing the survey and writing the manuscript. The grantor had no role in the study design, execution, analysis, or reporting. Greene and Pardo are employed by PRIME. Singh and Carden are employed by AMCP. Greene, Singh, Carden, and Pardo have no other disclosures. Lichtenstein received an honorarium from PRIME for serving as faculty for the continuing education program and has been a consultant for Pfizer, Cellceutix, and Merck.
Conflict of interest statement
The survey study reported in this article was part of a continuing education program funded by an independent educational grant, which was awarded by Sandoz, a Novartis Division, to PRIME Education. The Academy of Managed Care Pharmacy (AMCP) received grant funding from PRIME to assist in developing the survey and writing the manuscript. The grantor had no role in the study design, execution, analysis, or reporting. Greene and Pardo are employed by PRIME. Singh and Carden are employed by AMCP. Greene, Singh, Carden, and Pardo have no other disclosures. Lichtenstein received an honorarium from PRIME for serving as faculty for the continuing education program and has been a consultant for Pfizer, Cellceutix, and Merck.
Figures
Similar articles
-
Overcoming barriers to biosimilar adoption: real-world perspectives from a national payer and provider initiative.J Manag Care Spec Pharm. 2021 Aug;27(8):1129-1135. doi: 10.18553/jmcp.2021.27.8.1129. J Manag Care Spec Pharm. 2021. PMID: 34337986 Free PMC article.
-
AMCP Partnership Forum: Biosimilars--Ready, Set, Launch.J Manag Care Spec Pharm. 2016 Apr;22(4):434-40. doi: 10.18553/jmcp.2016.22.4.434. J Manag Care Spec Pharm. 2016. PMID: 27023697 Free PMC article.
-
Breaking Barriers: Essential Reforms for a Competitive and Affordable Biosimilars Market.Curr Rheumatol Rep. 2025 May 28;27(1):25. doi: 10.1007/s11926-025-01192-x. Curr Rheumatol Rep. 2025. PMID: 40434488 Review.
-
Biosimilar competition in the United States: statutory incentives, payers, and pharmacy benefit managers.Health Aff (Millwood). 2015 Feb;34(2):294-301. doi: 10.1377/hlthaff.2014.0482. Health Aff (Millwood). 2015. PMID: 25646110
-
Identification of Barriers Preventing Biosimiliar Oncology Medication Adoption.Medicina (Kaunas). 2022 Oct 27;58(11):1533. doi: 10.3390/medicina58111533. Medicina (Kaunas). 2022. PMID: 36363490 Free PMC article. Review.
Cited by
-
Biosimilar knowledge and viewpoints among Brazilian inflammatory bowel disease patients.Therap Adv Gastroenterol. 2021 May 18;14:17562848211013249. doi: 10.1177/17562848211013249. eCollection 2021. Therap Adv Gastroenterol. 2021. PMID: 34046083 Free PMC article.
-
Prescriber Perspectives on Biosimilar Adoption and Potential Role of Clinical Pharmacology: A Workshop Summary.Clin Pharmacol Ther. 2023 Jan;113(1):37-49. doi: 10.1002/cpt.2765. Epub 2022 Nov 4. Clin Pharmacol Ther. 2023. PMID: 36251545 Free PMC article. Review.
-
Patient's and Consultant's Views and Perceptions on Switching from an Originator Biologic to Biosimilar Medication: A Qualitative Study.Pharmacy (Basel). 2024 Apr 7;12(2):65. doi: 10.3390/pharmacy12020065. Pharmacy (Basel). 2024. PMID: 38668091 Free PMC article.
-
AMCP Partnership Forum: Biosimilars-policy, practice, and postmarketing surveillance to support treatment and coverage decisions.J Manag Care Spec Pharm. 2021 Oct;27(10):1503-1508. doi: 10.18553/jmcp.2021.21103. Epub 2021 Aug 28. J Manag Care Spec Pharm. 2021. PMID: 34459234 Free PMC article.
-
Innovative Formulation Strategies for Biosimilars: Trends Focused on Buffer-Free Systems, Safety, Regulatory Alignment, and Intellectual Property Challenges.Pharmaceuticals (Basel). 2025 Jun 17;18(6):908. doi: 10.3390/ph18060908. Pharmaceuticals (Basel). 2025. PMID: 40573303 Free PMC article. Review.
References
-
- Mulcahy AW, Hlavka JP, Case SR. Biosimilar cost savings in the United States: initial experience and future potential. Rand Health Q. 2018;7(4):3. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6075809/. Accessed March 26, 2019. - PMC - PubMed
-
- Leonard E, Wascovich M, Oskouei S, Gurz P, Carpenter D. Factors affecting health care provider knowledge and acceptance of biosimilar medicines: a systematic review. J Manag Care Spec Pharm. 2019;25(1):102-12. Available at: https://www.jmcp.org/doi/full/10.18553/jmcp.2019.25.1.102. - DOI - PMC - PubMed
-
- Jacobs I, Singh E, Sewell KL, Al-Sabbagh A, Shane LG. Patient attitudes and understanding about biosimilars: an international cross-sectional survey. Patient Prefer Adherence. 2016;10:937-48. Available at: https://www.dovepress.com/patient-attitudes-and-understanding-about-bios.... Accessed March 26, 2019. - PMC - PubMed
-
- Feagan BG, Lam G, Ma C, Lichtenstein GR. Systematic review: efficacy and safety of switching patients between reference and biosimilar infliximab. Aliment Pharmacol Ther. 2019;49(1):31-40. Available at: https://onlinelibrary.wiley.com/doi/epdf/10.1111/apt.14997. Accessed March 26, 2019. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical