The autophagic degradation of cytosolic pools of peroxisomal proteins by a new selective pathway
- PMID: 31007124
- PMCID: PMC6984484
- DOI: 10.1080/15548627.2019.1603546
The autophagic degradation of cytosolic pools of peroxisomal proteins by a new selective pathway
Abstract
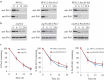
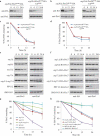
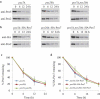
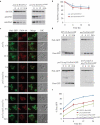
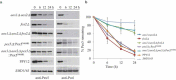
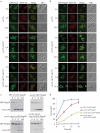
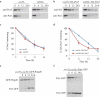
Damaged or redundant peroxisomes and their luminal cargoes are removed by pexophagy, a selective autophagy pathway. In yeasts, pexophagy depends mostly on the pexophagy receptors, such as Atg30 for Pichia pastoris and Atg36 for Saccharomyces cerevisiae, the autophagy scaffold proteins, Atg11 and Atg17, and the core autophagy machinery. In P. pastoris, the receptors for peroxisomal matrix proteins containing peroxisomal targeting signals (PTSs) include the PTS1 receptor, Pex5, and the PTS2 receptor and co-receptor, Pex7 and Pex20, respectively. These shuttling receptors are predominantly cytosolic and only partially peroxisomal. It remains unresolved as to whether, when and how the cytosolic pools of peroxisomal receptors, as well as the peroxisomal matrix proteins, are degraded under pexophagy conditions. These cytosolic pools exist both in normal and mutant cells impaired in peroxisome biogenesis. We report here that Pex5 and Pex7, but not Pex20, are degraded by an Atg30-independent, selective autophagy pathway. To enter this selective autophagy pathway, Pex7 required its major PTS2 cargo, Pot1. Similarly, the degradation of Pex5 was inhibited in cells missing abundant PTS1 cargoes, such as alcohol oxidases and Fox2 (hydratase-dehydrogenase-epimerase). Furthermore, in cells deficient in PTS receptors, the cytosolic pools of peroxisomal matrix proteins, such as Pot1 and Fox2, were also removed by Atg30-independent, selective autophagy, under pexophagy conditions. In summary, the cytosolic pools of PTS receptors and their cargoes are degraded via a pexophagy-independent, selective autophagy pathway under pexophagy conditions. These autophagy pathways likely protect cells from futile enzymatic reactions that could potentially cause the accumulation of toxic cytosolic products.Abbreviations: ATG: autophagy related; Cvt: cytoplasm to vacuole targeting; Fox2: hydratase-dehydrogenase-epimerase; PAGE: polyacrylamide gel electrophoresis; Pot1: thiolase; PMP: peroxisomal membrane protein; Pgk1: 3-phosphoglycerate kinase; PTS: peroxisomal targeting signal; RADAR: receptor accumulation and degradation in the absence of recycling; RING: really interesting new gene; SDS: sodium dodecyl sulphate; TCA, trichloroacetic acid; Ub: ubiquitin; UPS: ubiquitin-proteasome system Vid: vacuole import and degradation.
Keywords: Autophagy; PTS receptors; peroxisomal matrix proteins; peroxisome; pexophagy receptor.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
