Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function
- PMID: 31007132
- PMCID: PMC6589501
- DOI: 10.1089/ten.TEC.2018.0318
Fully Three-Dimensional Bioprinted Skin Equivalent Constructs with Validated Morphology and Barrier Function
Abstract
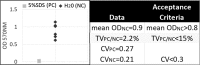
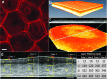
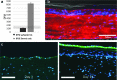
This article describes a method for the biofabrication of skin tissue equivalents in a multiwell plate format. The technique and results overcome shortcomings of previously published engineering methods, and show good architecture and barrier function from well to well; thus it may be used for compound functional testing and for the development of disease tissue models for screening.
Keywords: barrier function; bioprinting; high-throughput screening; keratinocytes; skin.
Conflict of interest statement
No competing financial interests exist.
Figures



References
-
- Bouwstra J.A. The skin barrier, a well-organized membrane. Colloids Surfaces 124, 403, 1997
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

