The nature, origin and modification of insoluble organic matter in chondrites, the possibly interstellar source of Earth's C and N
- PMID: 31007270
- PMCID: PMC6469876
- DOI: 10.1016/j.chemer.2017.01.007
The nature, origin and modification of insoluble organic matter in chondrites, the possibly interstellar source of Earth's C and N
Abstract
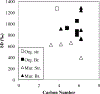
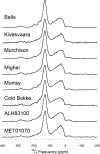
All chondrites accreted ~3.5 wt.% C in their matrices, the bulk of which was in a macromolecular solvent and acid insoluble organic material (IOM). Similar material to IOM is found in interplanetary dust particles (IDPs) and comets. The IOM accounts for almost all of the C and N in chondrites, and a significant fraction of the H. Chondrites and, to a lesser extent, comets were probably the major sources of volatiles for the Earth and the other terrestrial planets. Hence, IOM was both the major source of Earth's volatiles and a potential source of complex prebiotic molecules. Large enrichments in D and 15N, relative to the bulk solar isotopic compositions, suggest that IOM or its precursors formed in very cold, radiation-rich environments. Whether these environments were in the interstellar medium (ISM) or the outer Solar System is unresolved. Nevertheless, the elemental and isotopic compositions and functional group chemistry of IOM provide important clues to the origin(s) of organic matter in protoplanetary disks. IOM is modified relatively easily by thermal and aqueous processes, so that it can also be used to constrain the conditions in the solar nebula prior to chondrite accretion and the conditions in the chondrite parent bodies after accretion. Here we review what is known about the abundances, compositions and physical nature of IOM in the most primitive chondrites. We also discuss how the IOM has been modified by thermal metamorphism and aqueous alteration in the chondrite parent bodies, and how these changes may be used both as petrologic indicators of the intensity of parent body processing and as tools for classification. Finally, we critically assess the various proposed mechanisms for the formation of IOM in the ISM or Solar System.
Figures











References
-
- Abreu NM, Brearley AJ, 2004. Characterisation of matrix in the EET92042 CR2 carbonaceous chondrite: Insights into textural and mineralogical heterogeneity. Meteor. Planet. Sci 39, A12 (abstr.).
-
- Abreu NM, Brearley AJ, 2010. Early solar system processes recorded in the matrices of two highly pristine CR3 carbonaceous chondrites, MET 00426 and QUE 99177. Geochim. Cosmochim. Acta 74, 1146–1171.
-
- Abreu NM, Bullock ES, 2013. Opaque assemblages in CR2 Graves Nunataks (GRA) 06100 as indicators of shock-driven hydrothermal alteration in the CR chondrite parent body. Meteor. Planet. Sci 48, 2406–2429.
-
- Aléon J, Robert F, Chaussidon M, Marty B, 2003. Nitrogen isotopic composition of macromolecular organic matter in interplanetary dust particles. Geochim. Cosmochim. Acta 67, 3773–3783.
-
- Alexander CMO’D, Russell SS, Arden JW, Ash RD, Grady MM Pillinger CT, 1998. The origin of chondritic macromolecular organic matter: A carbon and nitrogen isotope study. Meteor. Planet. Sci 33, 603–622. - PubMed
