Photoactive Molecular-Based Devices, Machines and Materials: Recent Advances
- PMID: 31007574
- PMCID: PMC6472663
- DOI: 10.1002/ejic.201800923
Photoactive Molecular-Based Devices, Machines and Materials: Recent Advances
Abstract
Molecular and supramolecular-based systems and materials that can perform predetermined functions in response to light stimulation have been extensively studied in the past three decades. Their investigation continues to be a highly stimulating topic of chemical research, not only because of the inherent scientific value related to a bottom-up approach to functional nanostructures, but also for the prospective applications in diverse fields of technology and medicine. Light is an important tool in this context, as it can be conveniently used both for supplying energy to the system and for probing its states and transformations. In this microreview we recall some basic aspects of light-induced processes in (supra)molecular assemblies, and discuss their exploitation to implement novel functionalities with nanostructured devices, machines and materials. To this aim we illustrate a few examples from our own recent work, which are meant to illustrate the trends of current research in the field.
Keywords: Azobenzene; Molecular machines; Photochemistry; Quantum dots; Rotaxanes; Supramolecular chemistry.
Figures
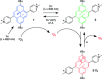
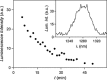
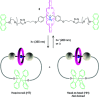
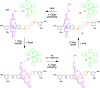
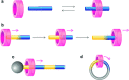
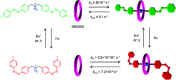
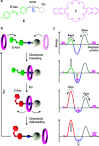

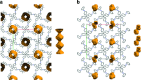
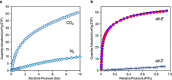
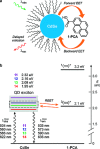
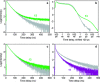
References
-
- Photobiology ‐ The Science of Life and Light (Ed. L. O. Björn), Springer, New York, 2008.
-
- Ramamurthy V. and Mondal B., J. Photochem. Photobiol. C, 2015, 23, 68.
-
- Ceroni P., Credi A. and Venturi M., Chem. Soc. Rev., 2014, 43, 4068. - PubMed
-
- Joachim C. and Launay J.‐P., Nouv. J. Chim., 1984, 8, 723.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
