Differential adhesion and fibrinolytic activity of mesenchymal stem cells from human bone marrow, placenta, and Wharton's jelly cultured in a fibrin hydrogel
- PMID: 31007888
- PMCID: PMC6460889
- DOI: 10.1177/2041731419840622
Differential adhesion and fibrinolytic activity of mesenchymal stem cells from human bone marrow, placenta, and Wharton's jelly cultured in a fibrin hydrogel
Abstract
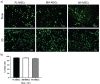
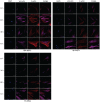
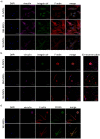
Mesenchymal stem cells isolated from different tissues should share associated markers and the capability to differentiate to mesodermal lineages. However, their behavior varies in specific microenvironments. Herein, adhesion and fibrinolytic activity of mesenchymal stem cells from placenta, bone marrow, and Wharton's jelly were evaluated in fibrin hydrogels prepared with nonpurified blood plasma and compared with two-dimensional cultures. Despite the source, mesenchymal stem cells adhered through focal adhesions positive for vinculin and integrin αV in two dimensions, while focal adhesions could not be detected in fibrin hydrogels. Moreover, some cells could not spread and stay rounded. The proportions of elongated and round phenotypes varied, with placenta mesenchymal stem cells having the lowest percentage of elongated cells (~10%). Mesenchymal stem cells degraded fibrin at distinct rates, and placenta mesenchymal stem cells had the strongest fibrinolytic activity, which was achieved principally through the plasminogen-plasmin axis. These findings might have clinical implications in tissue engineering and wound healing therapy.
Keywords: Human mesenchymal stem cells; adhesion; fibrin; fibrinolysis.
Conflict of interest statement
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures




References
-
- Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007; 13(6): 1299–1312. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

