Synthesis and Structure Revision of Symplocin A
- PMID: 31007934
- PMCID: PMC6474688
- DOI: 10.1039/C7QO00052A
Synthesis and Structure Revision of Symplocin A
Abstract
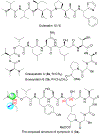
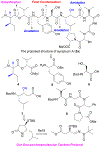
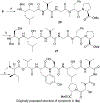
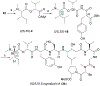
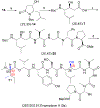
Symplocin A, a linear peptide possessing N-terminal N,N-dimethylisoleucine, statine, and valic acid residues, has been synthesized for the first time employing our previously established 'one-pot intramolecular tandem protocol'. Moreover, the stereochemistry of natural symplocin A was unambiguously revised through the confirmation by 1D NMR, 2D NMR, and HPLC comparisons with authentic natural product.
Figures

Similar articles
-
Symplocin A, a linear peptide from the Bahamian cyanobacterium Symploca sp. Configurational analysis of N,N-dimethylamino acids by chiral-phase HPLC of naphthacyl esters.J Nat Prod. 2012 Mar 23;75(3):425-31. doi: 10.1021/np200861n. Epub 2012 Feb 23. J Nat Prod. 2012. PMID: 22360587 Free PMC article.
-
First Total Synthesis, Structure Revision, and Natural History of the Smallest Cytochalasin: (+)-Periconiasin G.Chemistry. 2016 Oct 17;22(43):15257-15260. doi: 10.1002/chem.201603734. Epub 2016 Sep 9. Chemistry. 2016. PMID: 27556729
-
A Highly Stereoselective One-Pot Tandem Consecutive 1,4-Addition-Intramolecular 1,3-Dipolar Cycloaddition Strategy for the Construction of Functionalized Five- and Six-Membered Carbocycles(,)(1).J Org Chem. 1997 Feb 7;62(3):485-492. doi: 10.1021/jo961663v. J Org Chem. 1997. PMID: 11671438
-
Synthesis of the lantibiotic lactocin S using peptide cyclizations on solid phase.J Am Chem Soc. 2010 Jan 20;132(2):462-3. doi: 10.1021/ja9095945. J Am Chem Soc. 2010. PMID: 20017553
-
Screening of synthetic PDE-5 inhibitors and their analogues as adulterants: analytical techniques and challenges.J Pharm Biomed Anal. 2014 Jan;87:176-90. doi: 10.1016/j.jpba.2013.04.037. Epub 2013 May 6. J Pharm Biomed Anal. 2014. PMID: 23721687 Review.
Cited by
-
Isochalcogenourea-catalyzed asymmetric (4 + 2)-heterocycloadditions of allenoates.Chem Lett. 2024 Aug 23;53(9):upae168. doi: 10.1093/chemle/upae168. eCollection 2024 Sep 2. Chem Lett. 2024. PMID: 40621370 Free PMC article.
-
Asymmetric isochalcogenourea-catalysed (4 + 2)-cycloadditions of ortho-quinone methides and allenoates.Org Biomol Chem. 2025 Jan 22;23(4):827-834. doi: 10.1039/d4ob01855a. Org Biomol Chem. 2025. PMID: 39636302 Free PMC article.
-
N-tert-Butanesulfinyl imines in the asymmetric synthesis of nitrogen-containing heterocycles.Beilstein J Org Chem. 2021 May 12;17:1096-1140. doi: 10.3762/bjoc.17.86. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34093879 Free PMC article. Review.
References
-
- Hamada Y and Shioiri T, Chem. Rev. (Washington, DC, U. S.), 2005, 105, 4441; - PubMed
- Bionda N and Cudic P, Croat. Chem. Acta, 2011, 84, 315;
- Salvador-Reyes LA and Luesch H, Nat. Prod. Rep, 2015, 32, 478; - PMC - PubMed
- Vijayakumar S, Manogar P and Prabhu S, Biomed. Pharmacother, 2016, 83, 362. - PubMed
-
- Luesch H, Moore RE, Paul VJ, Mooberry SL and Corbett TH, J. Nat. Prod, 2001, 64, 907. - PubMed
-
- Gerber H-P, Koehn FE, Abraham RT, Nat. Prod. Rep, 2013, 30, 625; - PubMed
- Perez EA, Hillman DW, Fishkin PA, Krook JE, Tan WW, Kuriakose PA, Alberts SR and Dakhil SR, Investigational New Drugs, 2005, 23, 257; - PubMed
- Kindler HL, Tothy PK, Wolff R, McCormack RA, Abbruzzese JL, Mani S, Wade-Oliver KT and Vokes EE, Investigational New Drugs, 2005, 23, 489. - PubMed
