Phosphothreonine (pThr)-Based Multifunctional Peptide Catalysis for Asymmetric Baeyer-Villiger Oxidations of Cyclobutanones
- PMID: 31007966
- PMCID: PMC6467539
- DOI: 10.1021/acscatal.8b04132
Phosphothreonine (pThr)-Based Multifunctional Peptide Catalysis for Asymmetric Baeyer-Villiger Oxidations of Cyclobutanones
Abstract
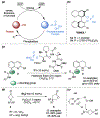
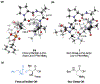
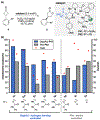
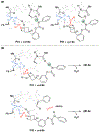
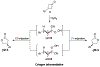
Biologically inspired phosphothreonine (pThr)-embedded peptides that function as chiral Brønsted acid catalysts for enantioselective Baeyer-Villiger oxidations (BV) of cyclobutanones with aqueous H2O2 are reported herein. Complementary to traditional BINOL-derived chiral phosphoric acids (CPAs), the functional diversity of the peptidic scaffold provides the opportunity for multiple points of contact with substrates via hydrogen bonding, and the ease of peptide synthesis facilitates rapid diversification of the catalyst structure, such that numerous unique peptide-based CPA catalysts have been prepared. Utilizing a hypothesis-driven design, we identified a pThr-based catalyst that contains an N-acylated diaminopropionic acid (Dap) residue, which achieves high enantioselectivity with catalyst loadings as low as 0.5 mol%. The power of peptide-based multi-site binding is further exemplified through reversal in the absolute stereochemical outcome upon repositioning of the substrate-directing group (ortho- to meta). Modifications to the i+3 residue (LDap to LPhe) lead to an observed enantiodivergence without inversion of any stereogenic center on the peptide catalyst, due to noncovalent interactions. Structure-selectivity and 1H-1H-ROESY studies revealed that the proposed hydrogen bonding interactions are essential for high levels of enantioinduction. The ability for the phosphopeptides to operate as multifunctional oxidation catalysts expands the scope of pThr catalysts and provides a framework for the future selective diversification of more complex substrates, including natural products.
Keywords: Baeyer–Villiger; asymmetric catalysis; chiral phosphoric acid; peptides; phosphothreonine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

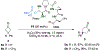

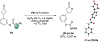
Similar articles
-
Phosphothreonine as a catalytic residue in peptide-mediated asymmetric transfer hydrogenations of 8-aminoquinolines.Angew Chem Int Ed Engl. 2015 Sep 14;54(38):11173-6. doi: 10.1002/anie.201505898. Epub 2015 Aug 5. Angew Chem Int Ed Engl. 2015. PMID: 26246129 Free PMC article.
-
Catalyst control over regio- and enantioselectivity in Baeyer-Villiger oxidations of functionalized ketones.J Am Chem Soc. 2014 Oct 8;136(40):14019-22. doi: 10.1021/ja508757g. Epub 2014 Sep 29. J Am Chem Soc. 2014. PMID: 25250713 Free PMC article.
-
Divergent Stereoselectivity in Phosphothreonine (pThr)-Catalyzed Reductive Aminations of 3-Amidocyclohexanones.J Org Chem. 2018 Apr 20;83(8):4491-4504. doi: 10.1021/acs.joc.8b00207. Epub 2018 Apr 5. J Org Chem. 2018. PMID: 29547285 Free PMC article.
-
Baeyer-Villiger oxidation: a promising tool for the synthesis of natural products: a review.RSC Adv. 2024 Jul 25;14(32):23423-23458. doi: 10.1039/d4ra03914a. eCollection 2024 Jul 19. RSC Adv. 2024. PMID: 39055269 Free PMC article. Review.
-
Transition Metal Catalysis in the Baeyer-Villiger Oxidation of Ketones.Angew Chem Int Ed Engl. 1998 May 18;37(9):1198-1209. doi: 10.1002/(SICI)1521-3773(19980518)37:9<1198::AID-ANIE1198>3.0.CO;2-Y. Angew Chem Int Ed Engl. 1998. PMID: 29711244 Review.
Cited by
-
Enantioselective Desymmetrization of Cyclobutanones: A Speedway to Molecular Complexity.Angew Chem Int Ed Engl. 2020 Apr 27;59(18):6964-6974. doi: 10.1002/anie.201910767. Epub 2020 Feb 19. Angew Chem Int Ed Engl. 2020. PMID: 31550067 Free PMC article. Review.
-
Disparate Catalytic Scaffolds for Atroposelective Cyclodehydration.J Am Chem Soc. 2019 Apr 24;141(16):6698-6705. doi: 10.1021/jacs.9b01911. Epub 2019 Apr 10. J Am Chem Soc. 2019. PMID: 30920223 Free PMC article.
-
Cu(ii)/SPDO complex catalyzed asymmetric Baeyer-Villiger oxidation of 2-arylcyclobutanones and its application for the total synthesis of eupomatilones 5 and 6.Chem Sci. 2022 Jun 23;13(28):8429-8435. doi: 10.1039/d2sc02079c. eCollection 2022 Jul 20. Chem Sci. 2022. PMID: 35919715 Free PMC article.
-
Data Science Enables the Development of a New Class of Chiral Phosphoric Acid Catalysts.Chem. 2023 Jun 8;9(6):1518-1537. doi: 10.1016/j.chempr.2023.02.020. Epub 2023 Mar 24. Chem. 2023. PMID: 37519827 Free PMC article.
-
Nickel-Catalyzed Asymmetric Domino Ring Opening/Cross-Coupling Reaction of Cyclobutanones via a Reductive Strategy.iScience. 2020 Apr 24;23(4):101017. doi: 10.1016/j.isci.2020.101017. Epub 2020 Apr 3. iScience. 2020. PMID: 32289735 Free PMC article.
References
-
- Westheimer FH Why Nature Chose Phosphates. Science 1987, 235, 1173–1178. - PubMed
-
- Selected reviews: Barford D; Das AK; Egloff M-P The Structure and Mechanism of Protein Phosphatases: Insights Into Catalysis and Regulation. Annu. Rev. Biophys. Biomol. Struct 1998, 27, 133–164. - PubMed
- Hunter T Signaling–2000 and Beyond. Cell 2000, 100, 113–127. - PubMed
- Cohen P The Regulation of Protein Function by Multisite Phosphorylation–A 25 Year Update. Trends Biochem. Sci 2000, 25, 596–601. - PubMed
-
- Yaffe MB; Elia AEH Phosphoserine/Threonine-Binding Domains. Curr. Opin. Cell Biol 2001, 13, 131–138. - PubMed
- Yaffe MB; Rittinger K; Volinia S; Caron PR; Aitken A; Leffers H; Gamblin SJ; Smerdon SJ; Cantley LC The Structural Basis for 14–3-3:Phosphopeptide Binding Specificity. Cell 1997, 91, 961–971. - PubMed
- Verdecia MA; Bowman ME; Lu KP; Hunter T; Noel JP Structural Basis for Phosphoserine-Proline Recognition by Group IV WW Domains. Nat. Struct. Mol. Biol 2000, 7, 639–643. - PubMed
-
- Seminal reports Uraguchi D; Terada M Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation. J. Am. Chem. Soc 2004, 126, 5356–5357. - PubMed
- Akiyama T; Itoh J; Yokota K; Fuchibe K Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid. Angew. Chem. Int. Ed 2004, 43, 1566–1568. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
