Phosphothreonine (pThr)-Based Multifunctional Peptide Catalysis for Asymmetric Baeyer-Villiger Oxidations of Cyclobutanones
- PMID: 31007966
- PMCID: PMC6467539
- DOI: 10.1021/acscatal.8b04132
Phosphothreonine (pThr)-Based Multifunctional Peptide Catalysis for Asymmetric Baeyer-Villiger Oxidations of Cyclobutanones
Abstract
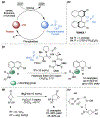
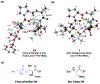
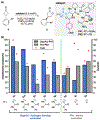
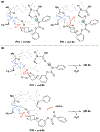
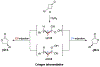
Biologically inspired phosphothreonine (pThr)-embedded peptides that function as chiral Brønsted acid catalysts for enantioselective Baeyer-Villiger oxidations (BV) of cyclobutanones with aqueous H2O2 are reported herein. Complementary to traditional BINOL-derived chiral phosphoric acids (CPAs), the functional diversity of the peptidic scaffold provides the opportunity for multiple points of contact with substrates via hydrogen bonding, and the ease of peptide synthesis facilitates rapid diversification of the catalyst structure, such that numerous unique peptide-based CPA catalysts have been prepared. Utilizing a hypothesis-driven design, we identified a pThr-based catalyst that contains an N-acylated diaminopropionic acid (Dap) residue, which achieves high enantioselectivity with catalyst loadings as low as 0.5 mol%. The power of peptide-based multi-site binding is further exemplified through reversal in the absolute stereochemical outcome upon repositioning of the substrate-directing group (ortho- to meta). Modifications to the i+3 residue (LDap to LPhe) lead to an observed enantiodivergence without inversion of any stereogenic center on the peptide catalyst, due to noncovalent interactions. Structure-selectivity and 1H-1H-ROESY studies revealed that the proposed hydrogen bonding interactions are essential for high levels of enantioinduction. The ability for the phosphopeptides to operate as multifunctional oxidation catalysts expands the scope of pThr catalysts and provides a framework for the future selective diversification of more complex substrates, including natural products.
Keywords: Baeyer–Villiger; asymmetric catalysis; chiral phosphoric acid; peptides; phosphothreonine.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

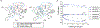
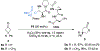

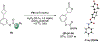
References
-
- Westheimer FH Why Nature Chose Phosphates. Science 1987, 235, 1173–1178. - PubMed
-
- Selected reviews: Barford D; Das AK; Egloff M-P The Structure and Mechanism of Protein Phosphatases: Insights Into Catalysis and Regulation. Annu. Rev. Biophys. Biomol. Struct 1998, 27, 133–164. - PubMed
- Hunter T Signaling–2000 and Beyond. Cell 2000, 100, 113–127. - PubMed
- Cohen P The Regulation of Protein Function by Multisite Phosphorylation–A 25 Year Update. Trends Biochem. Sci 2000, 25, 596–601. - PubMed
-
- Yaffe MB; Elia AEH Phosphoserine/Threonine-Binding Domains. Curr. Opin. Cell Biol 2001, 13, 131–138. - PubMed
- Yaffe MB; Rittinger K; Volinia S; Caron PR; Aitken A; Leffers H; Gamblin SJ; Smerdon SJ; Cantley LC The Structural Basis for 14–3-3:Phosphopeptide Binding Specificity. Cell 1997, 91, 961–971. - PubMed
- Verdecia MA; Bowman ME; Lu KP; Hunter T; Noel JP Structural Basis for Phosphoserine-Proline Recognition by Group IV WW Domains. Nat. Struct. Mol. Biol 2000, 7, 639–643. - PubMed
-
- Seminal reports Uraguchi D; Terada M Chiral Brønsted Acid-Catalyzed Direct Mannich Reactions via Electrophilic Activation. J. Am. Chem. Soc 2004, 126, 5356–5357. - PubMed
- Akiyama T; Itoh J; Yokota K; Fuchibe K Enantioselective Mannich-Type Reaction Catalyzed by a Chiral Brønsted Acid. Angew. Chem. Int. Ed 2004, 43, 1566–1568. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
