The assessment of the quality of randomized controlled trials published in Indian medical journals
- PMID: 31008074
- PMCID: PMC6463507
- DOI: 10.4103/picr.PICR_60_18
The assessment of the quality of randomized controlled trials published in Indian medical journals
Abstract
Aim: In this retrospective cross-sectional study, we sought to evaluate if the published randomized controlled trials (RCTs) reported in the year 2017 among the Indian medical journals (IMJs) complied with the Consolidated Standards of Reporting Trials (CONSORT) guidelines and identify domains where reporting could be improved.
Methods: A literature search was performed using PubMed and Google Scholar to identify all the IMJs that published RCTs in the year 2017. In the archives of the identified journals, the number of published RCTs was identified and the full text was obtained. We selected articles that stated RCT in abstract and title and that evaluated the safety and efficacy of all therapeutic and preventive interventions.
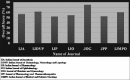
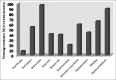
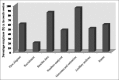
Results: A total of seven IMJs comprising of the Indian Journal of Anesthesia, Indian Journal of Dermatology, Venereology and Leprology, Indian Journal of Pharmacology, Indian Journal of Ophthalmology, Journal of Obstetrics and Gynaecology, Journal of Pharmacology and Pharmacotherapeutics, and Indian Journal of Medical and Pediatric Oncology that published a total of 84 RCTs were included. The mean compliance score of all the RCTs was 13.7 ± 2.66 (57%). Most RCTs had serious reporting deficiencies in the methodology and result sections.
Discussion: In spite of journals making it mandatory for prospective authors to comply with the CONSORT guidelines, it is intriguing that there continues to be significant lacunae in reporting RCTs adequately in most IMJs.
Conclusion: There is an urgent need to impart training to the medical community of our country in clinical research methods and reporting of RCTs.
Keywords: Compliance score; Consolidated Standards of Reporting Trials; Indian Medical Journals; Randomized Controlled Trials.
Conflict of interest statement
There are no conflicts of interest.
Figures
Similar articles
-
Assessment of Adherence to Consolidated Standards of Reporting Trials 2010 Guidelines of Randomized Controlled Trials Published in an Indian and International Pharmacology Journal From 2019 to 2023.Cureus. 2025 Mar 12;17(3):e80450. doi: 10.7759/cureus.80450. eCollection 2025 Mar. Cureus. 2025. PMID: 40225544 Free PMC article. Review.
-
Adherence of Randomized Controlled Trials to Consolidated Standards of Reporting Trials 2010 Guidelines: A Survey of Randomized Controlled Trials Published in 2011-2016 in 3 Periodontology Journals.J Evid Based Dent Pract. 2019 Sep;19(3):260-272. doi: 10.1016/j.jebdp.2019.04.001. Epub 2019 Apr 9. J Evid Based Dent Pract. 2019. PMID: 31732102
-
Rigor, reproducibility, and transparency of randomized controlled trials in obstetrics and gynecology.Am J Obstet Gynecol MFM. 2021 Nov;3(6):100450. doi: 10.1016/j.ajogmf.2021.100450. Epub 2021 Jul 26. Am J Obstet Gynecol MFM. 2021. PMID: 34325015 Free PMC article.
-
Standards for reporting randomized controlled trials in neurosurgery.J Neurosurg. 2011 Feb;114(2):280-5. doi: 10.3171/2010.8.JNS091770. Epub 2010 Nov 5. J Neurosurg. 2011. PMID: 21054137 Review.
-
Have CONSORT guidelines improved the quality of reporting of randomised controlled trials published in public health dentistry journals?Oral Health Prev Dent. 2013;11(2):95-103. doi: 10.3290/j.ohpd.a29359. Oral Health Prev Dent. 2013. PMID: 23534035
Cited by
-
Assessment of Reporting Quality of Drug-Related Randomized Controlled Trials Conducted in India and Published in MEDLINE-Indexed Indian Journals Over a Decade: A Systematic Review.Cureus. 2023 Jan 29;15(1):e34353. doi: 10.7759/cureus.34353. eCollection 2023 Jan. Cureus. 2023. PMID: 36874727 Free PMC article. Review.
-
Quality of the Systematic Reviews in Cochrane Gynecological Cancer Group and Their Understudied RCTs.J Obstet Gynaecol India. 2022 Aug;72(Suppl 1):346-351. doi: 10.1007/s13224-022-01655-6. Epub 2022 Apr 13. J Obstet Gynaecol India. 2022. PMID: 35928093 Free PMC article.
-
Assessment of Adherence to Consolidated Standards of Reporting Trials 2010 Guidelines of Randomized Controlled Trials Published in an Indian and International Pharmacology Journal From 2019 to 2023.Cureus. 2025 Mar 12;17(3):e80450. doi: 10.7759/cureus.80450. eCollection 2025 Mar. Cureus. 2025. PMID: 40225544 Free PMC article. Review.
-
Methodological quality and reporting of randomised controlled trials published in Indian medical journals.J Family Med Prim Care. 2022 Apr;11(4):1584-1585. doi: 10.4103/jfmpc.jfmpc_1741_21. Epub 2022 Mar 18. J Family Med Prim Care. 2022. PMID: 35516679 Free PMC article. No abstract available.
-
The utilization of systematic review evidence in formulating India's National Health Programme guidelines between 2007 and 2021.Health Policy Plan. 2023 Apr 11;38(4):435-453. doi: 10.1093/heapol/czad008. Health Policy Plan. 2023. PMID: 36715073 Free PMC article.
References
-
- Manchikanti L, Hirsch JA, Smith HS. Evidence-based medicine, systematic reviews, and guidelines in interventional pain management: Part 2: Randomized controlled trials. Pain Physician. 2008;11:717–73. - PubMed
-
- Tharyan P, George AT, Kirubakaran R, Barnabas JP. Reporting of methods was better in the clinical trials registry-India than in Indian journal publications. J Clin Epidemiol. 2013;66:10–22. - PubMed

