The Rat Medial Prefrontal Cortex Exhibits Flexible Neural Activity States during the Performance of an Odor Span Task
- PMID: 31008186
- PMCID: PMC6472939
- DOI: 10.1523/ENEURO.0424-18.2019
The Rat Medial Prefrontal Cortex Exhibits Flexible Neural Activity States during the Performance of an Odor Span Task
Abstract
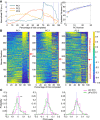
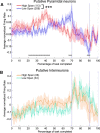
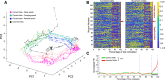
Medial prefrontal cortex (mPFC) activity is fundamental for working memory (WM), attention, and behavioral inhibition; however, a comprehensive understanding of the neural computations underlying these processes is still forthcoming. Toward this goal, neural recordings were obtained from the mPFC of awake, behaving rats performing an odor span task of WM capacity. Neural populations were observed to encode distinct task epochs and the transitions between epochs were accompanied by abrupt shifts in neural activity patterns. Putative pyramidal neuron activity increased earlier in the delay for sessions where rats achieved higher spans. Furthermore, increased putative interneuron activity was only observed at the termination of the delay thus indicating that local processing in inhibitory networks was a unique feature to initiate foraging. During foraging, changes in neural activity patterns associated with the approach to a novel odor, but not familiar odors, were robust. Collectively, these data suggest that distinct mPFC activity states underlie the delay, foraging, and reward epochs of the odor span task. Transitions between these states likely enables adaptive behavior in dynamic environments that place strong demands on the substrates of working memory.
Keywords: electrophysiology; multivariate statistics; odor; prefrontal cortex; pyramidal neuron; working memory.
Figures




Similar articles
-
Roles of the medial prefrontal cortex, mediodorsal thalamus, and their combined circuit for performance of the odor span task in rats: analysis of memory capacity and foraging behavior.Learn Mem. 2020 Jan 16;27(2):67-77. doi: 10.1101/lm.050195.119. Print 2020 Feb. Learn Mem. 2020. PMID: 31949038 Free PMC article.
-
Distinct Roles of Parvalbumin- and Somatostatin-Expressing Interneurons in Working Memory.Neuron. 2016 Nov 23;92(4):902-915. doi: 10.1016/j.neuron.2016.09.023. Epub 2016 Oct 13. Neuron. 2016. PMID: 27746132
-
Interactions between medial prefrontal cortex and dorsomedial striatum are necessary for odor span capacity in rats: role of GluN2B-containing NMDA receptors.Learn Mem. 2017 Sep 15;24(10):524-531. doi: 10.1101/lm.045419.117. Print 2017 Oct. Learn Mem. 2017. PMID: 28916627 Free PMC article.
-
Prefrontal Interneurons: Populations, Pathways, and Plasticity Supporting Typical and Disordered Cognition in Rodent Models.J Neurosci. 2022 Nov 9;42(45):8468-8476. doi: 10.1523/JNEUROSCI.1136-22.2022. J Neurosci. 2022. PMID: 36351822 Free PMC article. Review.
-
Prefrontal cortex and working memory processes.Neuroscience. 2006 Apr 28;139(1):251-61. doi: 10.1016/j.neuroscience.2005.07.003. Epub 2005 Dec 1. Neuroscience. 2006. PMID: 16325345 Review.
Cited by
-
Dorsomedial prefrontal cortex and hippocampus represent strategic context even while simultaneously changing representation throughout a task session.Neurobiol Learn Mem. 2020 May;171:107215. doi: 10.1016/j.nlm.2020.107215. Epub 2020 Apr 8. Neurobiol Learn Mem. 2020. PMID: 32276121 Free PMC article.
-
Distinct hippocampal-prefrontal neural assemblies coordinate memory encoding, maintenance, and recall.Curr Biol. 2023 Apr 10;33(7):1220-1236.e4. doi: 10.1016/j.cub.2023.02.029. Epub 2023 Mar 9. Curr Biol. 2023. PMID: 36898372 Free PMC article.
-
Roles of the medial prefrontal cortex, mediodorsal thalamus, and their combined circuit for performance of the odor span task in rats: analysis of memory capacity and foraging behavior.Learn Mem. 2020 Jan 16;27(2):67-77. doi: 10.1101/lm.050195.119. Print 2020 Feb. Learn Mem. 2020. PMID: 31949038 Free PMC article.
-
Dose-Dependent Regulation on Prefrontal Neuronal Working Memory by Dopamine D1 Agonists: Evidence of Receptor Functional Selectivity-Related Mechanisms.Front Neurosci. 2022 Jun 16;16:898051. doi: 10.3389/fnins.2022.898051. eCollection 2022. Front Neurosci. 2022. PMID: 35784852 Free PMC article.
-
Activity of CaMKII-expressing neurons in medial prefrontal cortex of male and female Long-Evans rats is necessary for encoding odor information and novelty recognition in an odor-based incidental memory test.Cereb Cortex. 2025 Aug 1;35(8):bhaf203. doi: 10.1093/cercor/bhaf203. Cereb Cortex. 2025. PMID: 40751661 Free PMC article.
References
-
- Akaike H (1974) A new look at the statistical model identification. IEEE Trans Automat Contr 19:716-723. 10.1109/TAC.1974.1100705 - DOI
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
