Effectiveness of monthly intravenous ibandronate injections in a real-world setting: Subgroup analysis of a postmarketing observational study
- PMID: 31008373
- PMCID: PMC6452926
- DOI: 10.1016/j.afos.2019.02.002
Effectiveness of monthly intravenous ibandronate injections in a real-world setting: Subgroup analysis of a postmarketing observational study
Abstract
Objectives: The favorable safety and consistent effectiveness of monthly intravenous (IV) ibandronate injections was demonstrated in a prospective, postmarketing, observational study in Japanese patients with osteoporosis. Here, we present subgroup analyses from the study.
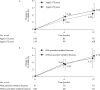
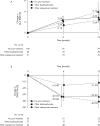
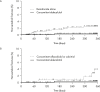
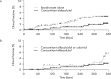
Methods: Lumbar spine (L2-4) bone mineral density (BMD) gains were assessed in the following subgroups: aged <75 or ≥75 years, absence or presence of vertebral fractures, previous bisphosphonate (BP) treatment, and concomitant versus naïve osteoporosis drug treatment. The cumulative incidence of fractures and relative change in bone turnover markers were also examined.
Results: Of 1062 enrolled patients, 1025 received monthly IV ibandronate 1 mg and were assessed for 12 months. BMD gains with ibandronate were comparable, irrespective of older age or prevalent fractures. Overall, 515 patients (50.2%) had previously received osteoporosis treatment; of these, 166 (16.1%) received other BPs. Mean BMD changes were 3.69% (95% confidence interval [CI], 0.89%-6.50%) in patients previously treated with other BPs, and 4.26% (95% CI, 2.88%-5.64%) in patients who had not received prior osteoporosis treatment. Among the 510 patients (49.7%) concomitantly prescribed active vitamin D drugs, mean BMD changes were 5.74% (95% CI, 2.53%-8.95%) with eldecalcitol versus 3.54% (95% CI, 1.98%-5.10%) with ibandronate alone. The lowest fracture incidence was observed with the combination of ibandronate and eldecalcitol, but differences between the subgroups were not statistically significant.
Conclusions: Monthly IV ibandronate demonstrated comparable BMD gains in the patient subgroups analyzed. Concomitant use of ibandronate with eldecalcitol showed a trend of higher BMD gains and lower fracture incidence than ibandronate alone.
Keywords: Eldecalcitol; Ibandronate; Japan; Observational study; Osteoporosis.
Figures

Similar articles
-
Safety and effectiveness of monthly intravenous ibandronate injections in a prospective, postmarketing, and observational study in Japanese patients with osteoporosis.Osteoporos Sarcopenia. 2018 Mar;4(1):22-28. doi: 10.1016/j.afos.2018.01.001. Epub 2018 Feb 17. Osteoporos Sarcopenia. 2018. PMID: 30775537 Free PMC article.
-
Clinical efficacy and safety of monthly oral ibandronate 100 mg versus monthly intravenous ibandronate 1 mg in Japanese patients with primary osteoporosis.Osteoporos Int. 2015 Nov;26(11):2685-93. doi: 10.1007/s00198-015-3175-1. Epub 2015 May 23. Osteoporos Int. 2015. PMID: 26001561 Free PMC article. Clinical Trial.
-
Update on monthly oral bisphosphonate therapy for the treatment of osteoporosis: focus on ibandronate 150 mg and risedronate 150 mg.Curr Med Res Opin. 2009 Dec;25(12):2951-60. doi: 10.1185/03007990903361307. Curr Med Res Opin. 2009. PMID: 19835464 Review.
-
Monthly oral ibandronate 100 mg is as effective as monthly intravenous ibandronate 1 mg in patients with various pathologies in the MOVEST study.J Bone Miner Metab. 2018 May;36(3):336-343. doi: 10.1007/s00774-017-0839-2. Epub 2017 Apr 7. J Bone Miner Metab. 2018. PMID: 28389932 Clinical Trial.
-
Once-monthly oral ibandronate in postmenopausal osteoporosis: translation and updated review.Clin Ther. 2009 Jul;31(7):1497-510. doi: 10.1016/j.clinthera.2009.07.018. Clin Ther. 2009. PMID: 19695399 Review.
Cited by
-
The therapeutic effect to eldecalcitol + bisphosphonate is superior to bisphosphonate alone in the treatment of osteoporosis: a meta-analysis.J Orthop Surg Res. 2020 Sep 9;15(1):390. doi: 10.1186/s13018-020-01896-z. J Orthop Surg Res. 2020. PMID: 32907639 Free PMC article.
-
Two-year effectiveness of zoledronic acid with or without eldecalcitol in Japanese patients with osteoporosis: A randomized prospective study.Osteoporos Sarcopenia. 2022 Jun;8(2):75-79. doi: 10.1016/j.afos.2022.05.001. Epub 2022 May 20. Osteoporos Sarcopenia. 2022. PMID: 35832418 Free PMC article.
References
-
- Eisman J.A., Civitelli R., Adami S., Czerwinski E., Recknor C., Prince R. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol. 2008;35:488–497. - PubMed
-
- Miller P.D., Recker R.R., Reginster J.Y., Riis B.J., Czerwinski E., Masanauskaite D. Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long-term extension study. Osteoporos Int. 2012;23:1747–1756. - PubMed
-
- Bianchi G., Czerwinski E., Kenwright A., Burdeska A., Recker R.R., Felsenberg D. Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int. 2012;23:1769–1778. - PubMed
-
- Harris S.T., Blumentals W.A., Miller P.D. Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin. 2008;24:237–245. - PubMed
LinkOut - more resources
Full Text Sources

