Effectiveness of monthly intravenous ibandronate injections in a real-world setting: Subgroup analysis of a postmarketing observational study
- PMID: 31008373
- PMCID: PMC6452926
- DOI: 10.1016/j.afos.2019.02.002
Effectiveness of monthly intravenous ibandronate injections in a real-world setting: Subgroup analysis of a postmarketing observational study
Abstract
Objectives: The favorable safety and consistent effectiveness of monthly intravenous (IV) ibandronate injections was demonstrated in a prospective, postmarketing, observational study in Japanese patients with osteoporosis. Here, we present subgroup analyses from the study.
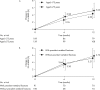
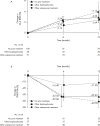
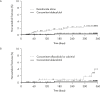
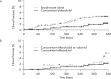
Methods: Lumbar spine (L2-4) bone mineral density (BMD) gains were assessed in the following subgroups: aged <75 or ≥75 years, absence or presence of vertebral fractures, previous bisphosphonate (BP) treatment, and concomitant versus naïve osteoporosis drug treatment. The cumulative incidence of fractures and relative change in bone turnover markers were also examined.
Results: Of 1062 enrolled patients, 1025 received monthly IV ibandronate 1 mg and were assessed for 12 months. BMD gains with ibandronate were comparable, irrespective of older age or prevalent fractures. Overall, 515 patients (50.2%) had previously received osteoporosis treatment; of these, 166 (16.1%) received other BPs. Mean BMD changes were 3.69% (95% confidence interval [CI], 0.89%-6.50%) in patients previously treated with other BPs, and 4.26% (95% CI, 2.88%-5.64%) in patients who had not received prior osteoporosis treatment. Among the 510 patients (49.7%) concomitantly prescribed active vitamin D drugs, mean BMD changes were 5.74% (95% CI, 2.53%-8.95%) with eldecalcitol versus 3.54% (95% CI, 1.98%-5.10%) with ibandronate alone. The lowest fracture incidence was observed with the combination of ibandronate and eldecalcitol, but differences between the subgroups were not statistically significant.
Conclusions: Monthly IV ibandronate demonstrated comparable BMD gains in the patient subgroups analyzed. Concomitant use of ibandronate with eldecalcitol showed a trend of higher BMD gains and lower fracture incidence than ibandronate alone.
Keywords: Eldecalcitol; Ibandronate; Japan; Observational study; Osteoporosis.
Figures

References
-
- Eisman J.A., Civitelli R., Adami S., Czerwinski E., Recknor C., Prince R. Efficacy and tolerability of intravenous ibandronate injections in postmenopausal osteoporosis: 2-year results from the DIVA study. J Rheumatol. 2008;35:488–497. - PubMed
-
- Miller P.D., Recker R.R., Reginster J.Y., Riis B.J., Czerwinski E., Masanauskaite D. Efficacy of monthly oral ibandronate is sustained over 5 years: the MOBILE long-term extension study. Osteoporos Int. 2012;23:1747–1756. - PubMed
-
- Bianchi G., Czerwinski E., Kenwright A., Burdeska A., Recker R.R., Felsenberg D. Long-term administration of quarterly IV ibandronate is effective and well tolerated in postmenopausal osteoporosis: 5-year data from the DIVA study long-term extension. Osteoporos Int. 2012;23:1769–1778. - PubMed
-
- Harris S.T., Blumentals W.A., Miller P.D. Ibandronate and the risk of non-vertebral and clinical fractures in women with postmenopausal osteoporosis: results of a meta-analysis of phase III studies. Curr Med Res Opin. 2008;24:237–245. - PubMed
LinkOut - more resources
Full Text Sources

