Generation of platelet-derived microparticles through the activation of the toll-like receptor 4
- PMID: 31008410
- PMCID: PMC6458467
- DOI: 10.1016/j.heliyon.2019.e01486
Generation of platelet-derived microparticles through the activation of the toll-like receptor 4
Abstract
Introduction: Infection from different bacterial may increase the risk of thrombosis and atherosclerosis risk by production and secretion of many proinflammatory factors. Human platelets have toll-like receptor 4 (TLR4), the principal receptor for lipopolysaccharide (LPS). The activation of platelet produces Platelet-derived Microparticles (PDMPs) measuring less than 1.0 micron (that are very abundant in circulation >90%), which are associated with the development of Cardiovascular Diseases (CVDs), the leading cause of death in the world.
Objectives: Experiments were designed to evaluate the generation of pro-thrombogenic microparticles in vitro on platelets via TLR4 activation.
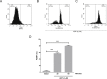
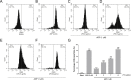
Methods: Platelet-rich plasma and washed platelets from healthy volunteers were incubated for the generation of PDMPs. The best source for the generation of microparticles was washed platelets. Then the washed platelets were incubated for 15 minutes with ultrapure Escherichia coli LPS (0-9 μg/mL) followed by activation with ADP (1 μM, subaggregant concentration), centrifuged for 60 minutes and analyzed by flow cytometry.
Results: Incubating platelets with LPS (9 μg/mL) and ADP (1 μM) produced a 34-fold increase in PDMPs generation. Finally, we evaluated this protocol to detect the inhibition of PDMPs generation, washed platelets were incubated with acetylsalicylic acid (10 μM) and an inhibition of 7.7-fold in PDMPs generation for activation of TLR4 was found.
Conclusion: A new and easy protocol for PDMPs generation and analysis by Flow Cytometry is established. In the future it could be used to determine the association of PDMPs with different pathologies.
Keywords: Biochemistry; Immunology.
Figures


Similar articles
-
Aggregation and microparticle production through toll-like receptor 4 activation in platelets from recently menopausal women.J Cardiovasc Pharmacol. 2009 Jul;54(1):57-62. doi: 10.1097/FJC.0b013e3181ab373d. J Cardiovasc Pharmacol. 2009. PMID: 19528814 Free PMC article.
-
Leukocyte- and platelet-derived microvesicle interactions following in vitro and in vivo activation of toll-like receptor 4 by lipopolysaccharide.PLoS One. 2011;6(9):e25504. doi: 10.1371/journal.pone.0025504. Epub 2011 Sep 26. PLoS One. 2011. PMID: 21966536 Free PMC article.
-
Standardization of a fast and effective method for the generation and detection of platelet-derived microparticles by a flow cytometer.Immunol Lett. 2018 Feb;194:79-84. doi: 10.1016/j.imlet.2018.01.003. Epub 2018 Jan 9. Immunol Lett. 2018. PMID: 29329679
-
Overview of regulatory frameworks on the national lot release of plasma-derived medicinal products in Korea.Biologicals. 2024 May;86:101768. doi: 10.1016/j.biologicals.2024.101768. Epub 2024 May 10. Biologicals. 2024. PMID: 38733709 Review.
-
Challenges for Plasma-Derived Medicinal Products.Transfus Med Hemother. 2023 Jan 18;50(2):116-122. doi: 10.1159/000528959. eCollection 2023 Apr. Transfus Med Hemother. 2023. PMID: 37066053 Free PMC article. Review.
Cited by
-
Platelet-Derived Microparticles are an Important Biomarker in Patients with Cancer-Associated Thrombosis.Int J Gen Med. 2019 Dec 31;12:491-497. doi: 10.2147/IJGM.S236166. eCollection 2019. Int J Gen Med. 2019. PMID: 32099444 Free PMC article.
References
-
- Gregg D., Goldschmidt-Clermont P.J. Platelets and cardiovascular disease. Circulation. 2003;108:e88. - PubMed
-
- Flierl U., Bauersachs J., Schafer A. Modulation of platelet and monocyte function by the chemokine fractalkine (CX3 CL1) in cardiovascular disease. Eur. J. Clin. Investig. 2015;45:624. - PubMed
-
- Leroyer A., Tedgui A., Boulanger C. Role of microparticles in atherothrombosis. J. Intern. Med. 2008;263:528. - PubMed
LinkOut - more resources
Full Text Sources

