Next generation physiologically based kinetic (NG-PBK) models in support of regulatory decision making
- PMID: 31008414
- PMCID: PMC6472623
- DOI: 10.1016/j.comtox.2018.11.002
Next generation physiologically based kinetic (NG-PBK) models in support of regulatory decision making
Abstract
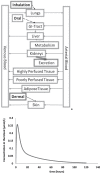
The fields of toxicology and chemical risk assessment seek to reduce, and eventually replace, the use of animals for the prediction of toxicity in humans. In this context, physiologically based kinetic (PBK) modelling based on in vitro and in silico kinetic data has the potential to a play significant role in reducing animal testing, by providing a methodology capable of incorporating in vitro human data to facilitate the development of in vitro to in vivo extrapolation of hazard information. In the present article, we discuss the challenges in: 1) applying PBK modelling to support regulatory decision making under the toxicology and risk-assessment paradigm shift towards animal replacement; 2) constructing PBK models without in vivo animal kinetic data, while relying solely on in vitro or in silico methods for model parameterization; and 3) assessing the validity and credibility of PBK models built largely using non-animal data. The strengths, uncertainties, and limitations of PBK models developed using in vitro or in silico data are discussed in an effort to establish a higher degree of confidence in the application of such models in a regulatory context. The article summarises the outcome of an expert workshop hosted by the European Commission Joint Research Centre (EC-JRC) - European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM), on "Physiologically-Based Kinetic modelling in risk assessment - reaching a whole new level in regulatory decision-making" held in Ispra, Italy, in November 2016, along with results from an international survey conducted in 2017 and recently reported activities occurring within the PBK modelling field. The discussions presented herein highlight the potential applications of next generation (NG)-PBK modelling, based on new data streams.
Keywords: In silico; In vitro; PBPK; PBTK; Physiologically based kinetic models; Toxicokinetics.
Figures

Similar articles
-
Systematic evaluation of high-throughput PBK modelling strategies for the prediction of intravenous and oral pharmacokinetics in humans.Arch Toxicol. 2024 Aug;98(8):2659-2676. doi: 10.1007/s00204-024-03764-9. Epub 2024 May 9. Arch Toxicol. 2024. PMID: 38722347 Free PMC article.
-
Towards best use and regulatory acceptance of generic physiologically based kinetic (PBK) models for in vitro-to-in vivo extrapolation (IVIVE) in chemical risk assessment.Arch Toxicol. 2022 Dec;96(12):3407-3419. doi: 10.1007/s00204-022-03356-5. Epub 2022 Sep 5. Arch Toxicol. 2022. PMID: 36063173 Free PMC article.
-
Development of a Combined In Vitro Physiologically Based Kinetic (PBK) and Monte Carlo Modelling Approach to Predict Interindividual Human Variation in Phenol-Induced Developmental Toxicity.Toxicol Sci. 2017 Jun 1;157(2):365-376. doi: 10.1093/toxsci/kfx054. Toxicol Sci. 2017. PMID: 28498972
-
Gaining acceptance in next generation PBK modelling approaches for regulatory assessments - An OECD international effort.Comput Toxicol. 2021 May;18:100163. doi: 10.1016/j.comtox.2021.100163. Comput Toxicol. 2021. PMID: 34027244 Free PMC article. Review.
-
A Systematic Review of Published Physiologically-based Kinetic Models and an Assessment of their Chemical Space Coverage.Altern Lab Anim. 2021 Sep;49(5):197-208. doi: 10.1177/02611929211060264. Epub 2021 Nov 26. Altern Lab Anim. 2021. PMID: 34836462 Free PMC article.
Cited by
-
Predictive Performance of Next Generation Physiologically Based Kinetic (PBK) Model Predictions in Rats Based on In Vitro and In Silico Input Data.Toxicol Sci. 2022 Feb 28;186(1):18-28. doi: 10.1093/toxsci/kfab150. Toxicol Sci. 2022. PMID: 34927682 Free PMC article.
-
Membrane transporter data to support kinetically-informed chemical risk assessment using non-animal methods: Scientific and regulatory perspectives.Environ Int. 2019 May;126:659-671. doi: 10.1016/j.envint.2019.03.003. Epub 2019 Mar 8. Environ Int. 2019. PMID: 30856453 Free PMC article. Review.
-
Systematic evaluation of high-throughput PBK modelling strategies for the prediction of intravenous and oral pharmacokinetics in humans.Arch Toxicol. 2024 Aug;98(8):2659-2676. doi: 10.1007/s00204-024-03764-9. Epub 2024 May 9. Arch Toxicol. 2024. PMID: 38722347 Free PMC article.
-
In Vitro-In Vivo Extrapolation by Physiologically Based Kinetic Modeling: Experience With Three Case Studies and Lessons Learned.Front Toxicol. 2022 Jul 18;4:885843. doi: 10.3389/ftox.2022.885843. eCollection 2022. Front Toxicol. 2022. PMID: 35924078 Free PMC article.
-
Investigation of parenteral nutrition-induced hepatotoxicity using human liver spheroid co-cultures.Arch Toxicol. 2024 Sep;98(9):3109-3126. doi: 10.1007/s00204-024-03773-8. Epub 2024 May 14. Arch Toxicol. 2024. PMID: 38740588 Free PMC article.
References
-
- Altenpohl A., Ciffroy P., Paini A., Radovnkovic A., Suciu N.A., Tanaka T., Tediosi A., Verdonck F. Standard documentation of exposure models: Merlin-Expo case study. Handbook Environ. Chem. 2018;57:59–76.
-
- Andersen, Krishnan, Quantitative Modeling in Toxicology: An Introduction Book Editor(s): Dr. Kannan Krishnan Dr Melvin E. Andersen. Wiley Online Library. First published: 30 March 2010, 2010. https://doi.org/10.1002/9780470686263.ch1.
-
- Armitage J.M., Wania F., Arnot J.A. Application of mass balance models and the chemical activity concept to facilitate the use of in vitro toxicity data for risk assessment. Environ. Sci. Technol. 2014;48(16):9770–9779. - PubMed
-
- Barton H.A., Chiu W.A., Setzer R.W., Andersen M.E., Bailer A.J., Bois F.Y. Characterizing uncertainty and variability in physiologically-based pharmacokinetic (PBPK) models: state of the science and needs for research and implementation. Toxicol. Sci. 2007;99(2):395–402. - PubMed
-
- Bell S.M., Chang X., Wambaugh J.F., Allen D.G., Bartels M., Brouwer K.L.R., Casey W.M., Choksi N., Ferguson S.S., Fraczkiewicz G., Jarabek A.M., Ke A., Lumen A., Lynn S.G., Paini A., Price P.S., Ring C., Simon T.W., Sipes N.S., Sprankle C.S., Strickland J., Troutman J., Wetmore B.A., Kleinstreuer N.C. In vitro to in vivo extrapolation for high throughput prioritization and decision making. Toxicol. In Vitro. 2018 Mar;47:213–227. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
