Between Perfectly Critical and Fully Irregular: A Reverberating Model Captures and Predicts Cortical Spike Propagation
- PMID: 31008508
- PMCID: PMC6519697
- DOI: 10.1093/cercor/bhz049
Between Perfectly Critical and Fully Irregular: A Reverberating Model Captures and Predicts Cortical Spike Propagation
Abstract
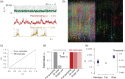
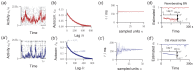
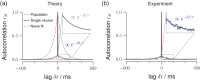
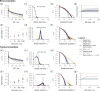
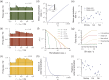
Knowledge about the collective dynamics of cortical spiking is very informative about the underlying coding principles. However, even most basic properties are not known with certainty, because their assessment is hampered by spatial subsampling, i.e., the limitation that only a tiny fraction of all neurons can be recorded simultaneously with millisecond precision. Building on a novel, subsampling-invariant estimator, we fit and carefully validate a minimal model for cortical spike propagation. The model interpolates between two prominent states: asynchronous and critical. We find neither of them in cortical spike recordings across various species, but instead identify a narrow "reverberating" regime. This approach enables us to predict yet unknown properties from very short recordings and for every circuit individually, including responses to minimal perturbations, intrinsic network timescales, and the strength of external input compared to recurrent activation "thereby informing about the underlying coding principles for each circuit, area, state and task.
Keywords: balanced state; criticality; perturbations; timescales.
© The Author(s) 2019. Published by Oxford University Press.
Figures
Similar articles
-
Criticality predicts maximum irregularity in recurrent networks of excitatory nodes.PLoS One. 2017 Aug 17;12(8):e0182501. doi: 10.1371/journal.pone.0182501. eCollection 2017. PLoS One. 2017. PMID: 28817580 Free PMC article.
-
Spontaneous cortical activity is transiently poised close to criticality.PLoS Comput Biol. 2017 May 24;13(5):e1005543. doi: 10.1371/journal.pcbi.1005543. eCollection 2017 May. PLoS Comput Biol. 2017. PMID: 28542191 Free PMC article.
-
Inferring collective dynamical states from widely unobserved systems.Nat Commun. 2018 Jun 13;9(1):2325. doi: 10.1038/s41467-018-04725-4. Nat Commun. 2018. PMID: 29899335 Free PMC article.
-
Space-Time Dynamics of Membrane Currents Evolve to Shape Excitation, Spiking, and Inhibition in the Cortex at Small and Large Scales.Neuron. 2017 Jun 7;94(5):934-942. doi: 10.1016/j.neuron.2017.04.038. Neuron. 2017. PMID: 28595049 Review.
-
Neural dynamics in cortical networks--precision of joint-spiking events.Novartis Found Symp. 2001;239:193-204; discussion 204-7, 234-40. doi: 10.1002/0470846674.ch15. Novartis Found Symp. 2001. PMID: 11529312 Review.
Cited by
-
Propagation of activity through the cortical hierarchy and perception are determined by neural variability.Nat Neurosci. 2023 Sep;26(9):1584-1594. doi: 10.1038/s41593-023-01413-5. Epub 2023 Aug 28. Nat Neurosci. 2023. PMID: 37640911 Free PMC article.
-
MR. Estimator, a toolbox to determine intrinsic timescales from subsampled spiking activity.PLoS One. 2021 Apr 29;16(4):e0249447. doi: 10.1371/journal.pone.0249447. eCollection 2021. PLoS One. 2021. PMID: 33914774 Free PMC article.
-
Sampling effects and measurement overlap can bias the inference of neuronal avalanches.PLoS Comput Biol. 2022 Nov 29;18(11):e1010678. doi: 10.1371/journal.pcbi.1010678. eCollection 2022 Nov. PLoS Comput Biol. 2022. PMID: 36445932 Free PMC article.
-
Subsampled Directed-Percolation Models Explain Scaling Relations Experimentally Observed in the Brain.Front Neural Circuits. 2021 Jan 15;14:576727. doi: 10.3389/fncir.2020.576727. eCollection 2020. Front Neural Circuits. 2021. PMID: 33519388 Free PMC article.
-
Brain criticality predicts individual levels of inter-areal synchronization in human electrophysiological data.Nat Commun. 2023 Aug 7;14(1):4736. doi: 10.1038/s41467-023-40056-9. Nat Commun. 2023. PMID: 37550300 Free PMC article.
References
-
- Atick JJ. 1992. Could information theory provide an ecological theory of sensory processing? Network-Comp Neural. 3(2):213–251. - PubMed
-
- Bak P, Tang C, Wiesenfeld K. 1987. Self-organized criticality: an explanation of the 1/f noise. Phys Rev Lett. 59(4):381–384. - PubMed
-
- Barlow HB. 2012. Possible principles underlying the transformations of sensory messages In: Rosenblith WA, editor. Sensory communication. Cambridge, MA: The MIT Press; p. 217–234.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

