Proteolysis mediated by the membrane-integrated ATP-dependent protease FtsH has a unique nonlinear dependence on ATP hydrolysis rates
- PMID: 31008538
- PMCID: PMC6567685
- DOI: 10.1002/pro.3629
Proteolysis mediated by the membrane-integrated ATP-dependent protease FtsH has a unique nonlinear dependence on ATP hydrolysis rates
Abstract
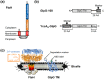
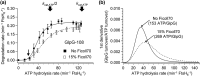
ATPases associated with diverse cellular activities (AAA+) proteases utilize ATP hydrolysis to actively unfold native or misfolded proteins and translocate them into a protease chamber for degradation. This basic mechanism yields diverse cellular consequences, including the removal of misfolded proteins, control of regulatory circuits, and remodeling of protein conformation. Among various bacterial AAA+ proteases, FtsH is only membrane-integrated and plays a key role in membrane protein quality control. Previously, we have shown that FtsH has substantial unfoldase activity for degrading membrane proteins overcoming a dual energetic burden of substrate unfolding and membrane dislocation. Here, we asked how efficiently FtsH utilizes ATP hydrolysis to degrade membrane proteins. To answer this question, we measured degradation rates of the model membrane substrate GlpG at various ATP hydrolysis rates in the lipid bilayers. We find that the dependence of degradation rates on ATP hydrolysis rates is highly nonlinear: (i) FtsH cannot degrade GlpG until it reaches a threshold ATP hydrolysis rate; (ii) after exceeding the threshold, the degradation rates steeply increase and saturate at the ATP hydrolysis rates far below the maxima. During the steep increase, FtsH efficiently utilizes ATP hydrolysis for degradation, consuming only 40-60% of the total ATP cost measured at the maximal ATP hydrolysis rates. This behavior does not fundamentally change against water-soluble substrates as well as upon addition of the macromolecular crowding agent Ficoll 70. The Hill analysis shows that the nonlinearity stems from coupling of three to five ATP hydrolysis events to degradation, which represents unique cooperativity compared to other AAA+ proteases including ClpXP, HslUV, Lon, and proteasomes.
Keywords: AAA+ protease; ATP hydrolysis rate; FtsH; cooperativity; membrane protein degradation; membrane protein folding; membrane protein quality control; steric trapping.
© 2019 The Protein Society.
Conflict of interest statement
The authors declare that there is no conflict of interest related to this work.
Figures



References
-
- Gottesman S (1996) Proteases and their targets in Escherichia coli . Annu Rev Genet 30:465–506. - PubMed
-
- Wickner S, Maurizi MR, Gottesman S (1999) Posttranslational quality control: folding, refolding, and degrading proteins. Science 286:1888–1893. - PubMed
-
- Gottesman S, Wickner S, Maurizi MR (1997) Protein quality control: triage by chaperones and proteases. Genes Dev 11:815–823. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

