Lactation induces increased IPSC bursting in oxytocinergic neurons
- PMID: 31008554
- PMCID: PMC6475881
- DOI: 10.14814/phy2.14047
Lactation induces increased IPSC bursting in oxytocinergic neurons
Abstract
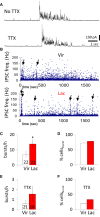
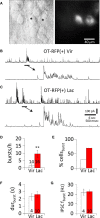
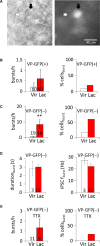
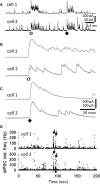
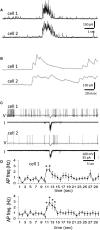
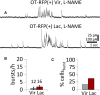
Hypothalamic magnocellular neurosecretory cells (MNCs) undergo dramatic structural reorganization during lactation in female rats that is thought to contribute to the pulsatile secretion of oxytocin critical for milk ejection. MNCs from male rats generate robust bursts of GABAergic synaptic currents, a subset of which are onset-synchronized between MNC pairs, but the functional role of the IPSC bursts is not known. To determine the physiological relevance of IPSC bursts, we compared MNCs from lactating and non-lactating female rats using whole-cell recordings in brain slices. We recorded a sixfold increase in the incidence of IPSC bursts in oxytocin (OT)-MNCs from lactating rats compared to non-lactating rats, whereas there was no change in IPSC bursts in vasopressin (VP)-MNCs. Synchronized bursts of IPSCs were observed in pairs of MNCs in slices from lactating rats. Our data indicate, therefore, that IPSC bursts are upregulated specifically in OT-MNCs during lactation, and may, therefore, contribute via rebound depolarization to the spike trains in OT neurons that lead to reflex milk ejection.
Keywords: GABA; IPSC; burst; lactation; oxytocin; synchronization.
© 2019 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
None declared.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

