Clinical and Molecular Phenotyping in Scleromyxedema Pretreatment and Posttreatment With Intravenous Immunoglobulin
- PMID: 31008568
- PMCID: PMC6810715
- DOI: 10.1002/acr.23908
Clinical and Molecular Phenotyping in Scleromyxedema Pretreatment and Posttreatment With Intravenous Immunoglobulin
Abstract
Objective: Scleromyxedema (SMX) is a rare systemic sclerosis mimic that often responds to intravenous immunoglobulin (IVIG) therapy, yet the resulting clinical and biochemical changes have not been well characterized. To better understand the pathogenesis of the disease and the efficacy of IVIG, we sought to explore whether IVIG would introduce a measurable biologic effect corresponding with clinical improvement.
Methods: Fifteen patients with SMX were recruited for the study. Clinical information and peripheral blood mononuclear cells for flow cytometry were obtained immediately before and again 1-2 weeks after patients received IVIG therapy. Ten patients also underwent skin biopsies for gene expression analysis both before and after IVIG therapy. Clinical data included measures of skin involvement (modification of the modified Rodnan skin thickness score [MMRSS] and percentage of body surface area) and several patient-reported outcome measures assessing patients' skin.
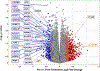
Results: Posttreatment, the average MMRSS score decreased from mean ± SD 13.6 ± 2.6 to 10.3 ± 1.9; P = 0.003. There were also significant improvements in skin flexibility (mean ± SD 5.4 ± 0.8 to 3.2 ± 0.7; P = 0.003) and softening (mean ± SD 4.9 ± 0.9 to 2.6 ± 0.6; P = 0.022). Baseline levels of Tc17 cells (CD8+CCR6+CXCR3+CCR4-) correlated with the extent of skin involvement as measured by MMRSS pretreatment (r = 0.69, P = 0.012) and decreased after IVIG therapy (mean ± SD 3.4% ± 3.2% to 1.3% ± 1.7%; P = 0.008). Posttreatment analysis of RNA in skin tissue revealed a decrease in gene expression of transforming growth factor β (TGFβ) cytokines as well as several interferon-inducible proteins.
Conclusion: This open-label study further supports the evidence that patients with SMX respond both objectively and subjectively to IVIG therapy. Biologic studies suggest a role for T lymphocytes in the pathogenesis of the disease and reveal the potential significance of TGFβ and interferon pathways.
© 2019, American College of Rheumatology.
Figures


References
-
- Hummers LK Scleromyxedema. Curr Opin Rheumatol 2014; 26:658–662. - PubMed
-
- Bogner RR, Wetter DA, & Dingli D Scleromyxedema. Internal Medicine (Tokyo, Japan) 2014;53:2561–2. - PubMed
-
- Rongioletti F, Merlo G, Cinotti E, Fausti V, Cozzani E, Cribier B, et al. Scleromyxedema: a multicenter study of characteristics, comorbidities, course, and therapy in 30 patients. J Am Acad Dermatol 2013;69:66–72. - PubMed
-
- Fleming KE, Virmani D, Sutton E, Langley R, Corbin J, Pasternak S, & Walsh NM Scleromyxedema and the dermato-neuro syndrome: case report and review of the literature. J Cutan Pathol 2012;39:508–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

