A major interspecies difference in the ionic selectivity of megakaryocyte Ca2+-activated channels sensitive to the TMEM16F inhibitor CaCCinh-A01
- PMID: 31008669
- PMCID: PMC6816474
- DOI: 10.1080/09537104.2019.1595560
A major interspecies difference in the ionic selectivity of megakaryocyte Ca2+-activated channels sensitive to the TMEM16F inhibitor CaCCinh-A01
Abstract
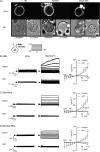
TMEM16F is a surface membrane protein critical for platelet procoagulant activity, which exhibits both phospholipid scramblase and ion channel activities following sustained elevation of cytosolic Ca2+. The extent to which the ionic permeability of TMEM16F is important for platelet scramblase responses remains controversial. To date, only one study has reported the electrophysiological properties of TMEM16F in cells of platelet/megakaryocyte lineage, which observed cation-selectivity within excised patch recordings from murine marrow-derived megakaryocytes. This contrasts with reports using whole-cell recordings that describe this channel as displaying either selectivity for anions or being relatively non-selective amongst the major physiological monovalent ions. We have studied TMEM16F expression and channel activity in primary rat and mouse megakaryocytes and the human erythroleukemic (HEL) cell line that exhibits megakaryocytic surface markers. Immunocytochemical analysis was consistent with surface TMEM16F expression in cells from all three species. Whole-cell recordings in the absence of K+-selective currents revealed an outwardly rectifying conductance activated by a high intracellular Ca2+ concentration in all three species. These currents appeared after 5-6 minutes and were blocked by CaCCinh-A01, properties typical of TMEM16F. Ion substitution experiments showed that the underlying conductance was predominantly Cl--permeable in rat megakaryocytes and HEL cells, yet non-selective between monovalent anions and cations in mouse megakaryocytes. In conclusion, the present study further highlights the difference in ionic selectivity of TMEM16F in platelet lineage cells of the mouse compared to other mammalian species. This provides additional support for the ionic "leak" hypothesis that the scramblase activity of TMEM16F does not rely upon its ability to conduct ions of a specific type.
Keywords: Calcium; Scott syndrome; TMEM16F; megakaryocyte; phospholipid scrambling; platelet.
Figures

References
-
- Zwaal RF, Schroit AJ.. Pathophysiologic implications of membrane phospholipid asymmetry in blood cells. Blood 1997;89(4):1121–1132. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
