Postvaccination Fever Response Rates in Children Derived Using the Fever Coach Mobile App: A Retrospective Observational Study
- PMID: 31008712
- PMCID: PMC6658305
- DOI: 10.2196/12223
Postvaccination Fever Response Rates in Children Derived Using the Fever Coach Mobile App: A Retrospective Observational Study
Erratum in
-
Correction: Postvaccination Fever Response Rates in Children Derived Using the Fever Coach Mobile App: A Retrospective Observational Study.JMIR Mhealth Uhealth. 2020 May 7;8(5):e18921. doi: 10.2196/18921. JMIR Mhealth Uhealth. 2020. PMID: 32379698 Free PMC article.
Abstract
Background: Postvaccination fever is a mild adverse event that naturally improves without complications, but is highly prevalent and can be accompanied by febrile convulsions in some cases. These adverse effects may cause parents to delay or avoid vaccinating their children.
Objective: This study aimed to identify postvaccination fever patterns and the ability of antipyretics to affect changes in these patterns from data collected from a mobile app named Fever Coach.
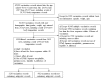
Methods: Data provided by parents of feverish children derived from a mobile app, Fever Coach, were used to identify postvaccination fever patterns according to vaccinations and the use of antipyretic drugs. We selected single vaccination records that contained five or more body temperature readings performed within 48 hours of vaccination, and we analyzed postvaccination fever onset, offset, duration, and maximum body temperature. Through observing the postvaccination fever response to vaccination, we identified the effects of antipyretic drugs on postvaccination fever onset, offset, and duration times; the extent of fever; and the rate of decline. We also performed logistic regression analysis to determine demographic variables (age, weight, and sex) involved in relatively high fevers (body temperature ≥39°C).
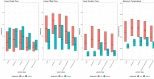
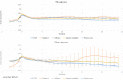
Results: The total number of Fever Coach users was 25,037, with 3834 users having entered single vaccination records, including 4448 vaccinations and 55,783 body temperature records. Most records were obtained from children receiving the following vaccinations: pneumococcus (n=2069); Japanese encephalitis (n=911); influenza (n=669); diphtheria, tetanus, and pertussis (n=403); and hepatitis A (n=252). According to the 4448 vaccination records, 3427 (77.05%) children had taken antipyretic drugs, and 3238 (89.15%) children took antibiotics at body temperatures above 38°C. The number of children taking antipyretics at a body temperature of 38°C was more than four times that of those taking antipyretics at 37.9°C (307 vs 67 cases). The number of instances in which this temperature threshold was reached was more than four times greater than the number when the temperature was 37.9°C. A comparative analysis of antipyretic and nonantipyretic cases showed there was no difference in onset time; however, offset and duration times were significantly shorter in nonantipyretic cases than in antipyretic cases (P<.001). In nonantipyretic cases, offset times and duration times were 9.9 and 10.1 hours shorter, respectively, than in antipyretic cases. Body temperatures also decreased faster in nonantipyretic cases. Influenza vaccine-associated fevers lasted relatively longer, whereas pneumococcus vaccine-associated fevers were relatively short-lived.
Conclusions: These findings suggest that postvaccination fever has its own fever pattern, which is dependent on vaccine type and the presence of antipyretic drugs, and that postvaccination temperature monitoring may ease fever phobia and reduce the unnecessary use of antipyretics in medical care.
Keywords: digital health care; mobile app; patient-generated health data; postvaccination fever; vaccination.
©Sang Hyun Ahn, Jooho Zhiang, Hyery Kim, Seyun Chang, Jaewon Shin, Myeongchan Kim, Yura Lee, Jae-Ho Lee, Yu Rang Park. Originally published in JMIR Mhealth and Uhealth (http://mhealth.jmir.org), 22.04.2019.
Conflict of interest statement
Conflicts of Interest: Seyun Chang, Jaewon Shin, and Myeongchan Kim are employees of Mobile Doctor Co, Ltd. All other authors declare no conflicts of interest.
Figures



Similar articles
-
Comparative Analysis of Single and Combined Antipyretics Using Patient-Generated Health Data: Retrospective Observational Study.JMIR Mhealth Uhealth. 2021 May 26;9(5):e21668. doi: 10.2196/21668. JMIR Mhealth Uhealth. 2021. PMID: 34037528 Free PMC article.
-
Impact of Fever and Antipyretic Use on Influenza Vaccine Immune Reponses in Children.Pediatr Infect Dis J. 2018 Oct;37(10):971-975. doi: 10.1097/INF.0000000000001949. Pediatr Infect Dis J. 2018. PMID: 29465480
-
The Fever Coach Mobile App for Participatory Influenza Surveillance in Children: Usability Study.JMIR Mhealth Uhealth. 2019 Oct 17;7(10):e14276. doi: 10.2196/14276. JMIR Mhealth Uhealth. 2019. PMID: 31625946 Free PMC article.
-
Combined and alternating paracetamol and ibuprofen therapy for febrile children.Evid Based Child Health. 2014 Sep;9(3):675-729. doi: 10.1002/ebch.1978. Evid Based Child Health. 2014. PMID: 25236309 Review.
-
[Management of fever in children younger then 3 years].J Pharm Belg. 2010 Sep;(3):53-7. J Pharm Belg. 2010. PMID: 21090380 Review. French.
Cited by
-
Association between the side effect induced by COVID-19 vaccines and the immune regulatory gene polymorphism.Front Immunol. 2022 Oct 26;13:941497. doi: 10.3389/fimmu.2022.941497. eCollection 2022. Front Immunol. 2022. PMID: 36389676 Free PMC article.
-
A predictive model to estimate fever after receipt of the second dose of Pfizer-BioNTech coronavirus disease 2019 vaccine: An observational cohort study.Health Sci Rep. 2022 Jul 20;5(4):e742. doi: 10.1002/hsr2.742. eCollection 2022 Jul. Health Sci Rep. 2022. PMID: 35873402 Free PMC article.
-
Management of acute fever in children: Consensus recommendations for community and primary healthcare providers in sub-Saharan Africa.Afr J Emerg Med. 2021 Jun;11(2):283-296. doi: 10.1016/j.afjem.2020.11.004. Epub 2021 Apr 10. Afr J Emerg Med. 2021. PMID: 33912381 Free PMC article. Review.
References
-
- World Health Organization. [2019-03-26]. WHO recommendations for routine immunization-summary tables https://www.who.int/immunization/policy/immunization_tables/en/
-
- Kelso JM, Greenhawt MJ, Li JT, Nicklas RA, Bernstein DI, Blessing-Moore J, Cox L, Khan D, Lang DM, Oppenheimer J, Portnoy JM, Randolph CR, Schuller DE, Spector SL, Tilles SA, Wallace D. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012 Jul;130(1):25–43. doi: 10.1016/j.jaci.2012.04.003.S0091-6749(12)00600-8 - DOI - PubMed
-
- Zhou W, Pool V, Iskander JK, English-Bullard R, Ball R, Wise RP, Haber P, Pless RP, Mootrey G, Ellenberg SS, Braun MM, Chen RT. Surveillance for safety after immunization: Vaccine Adverse Event Reporting System (VAERS)--United States, 1991-2001. MMWR Surveill Summ. 2003 Jan 24;52(1):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/ss5201a1.htm - PubMed
-
- Jampel HD, Duff GW, Gershon RK, Atkins E, Durum SK. Fever and immunoregulation. III. Hyperthermia augments the primary in vitro humoral immune response. J Exp Med. 1983 Apr 01;157(4):1229–1238. http://jem.rupress.org/cgi/pmidlookup?view=long&pmid=6220108 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

