Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons
- PMID: 31008816
- PMCID: PMC6669900
- DOI: 10.1097/j.pain.0000000000001488
Adenosine A3 receptor activation inhibits pronociceptive N-type Ca2+ currents and cell excitability in dorsal root ganglion neurons
Abstract
Recently, studies have focused on the antihyperalgesic activity of the A3 adenosine receptor (A3AR) in several chronic pain models, but the cellular and molecular basis of this effect is still unknown. Here, we investigated the expression and functional effects of A3AR on the excitability of small- to medium-sized, capsaicin-sensitive, dorsal root ganglion (DRG) neurons isolated from 3- to 4-week-old rats. Real-time quantitative polymerase chain reaction experiments and immunofluorescence analysis revealed A3AR expression in DRG neurons. Patch-clamp experiments demonstrated that 2 distinct A3AR agonists, Cl-IB-MECA and the highly selective MRS5980, inhibited Ca-activated K (KCa) currents evoked by a voltage-ramp protocol. This effect was dependent on a reduction in Ca influx via N-type voltage-dependent Ca channels, as Cl-IB-MECA-induced inhibition was sensitive to the N-type blocker PD173212 but not to the L-type blocker, lacidipine. The endogenous agonist adenosine also reduced N-type Ca currents, and its effect was inhibited by 56% in the presence of A3AR antagonist MRS1523, demonstrating that the majority of adenosine's effect is mediated by this receptor subtype. Current-clamp recordings demonstrated that neuronal firing of rat DRG neurons was also significantly reduced by A3AR activation in a MRS1523-sensitive but PD173212-insensitive manner. Intracellular Ca measurements confirmed the inhibitory role of A3AR on DRG neuronal firing. We conclude that pain-relieving effects observed on A3AR activation could be mediated through N-type Ca channel block and action potential inhibition as independent mechanisms in isolated rat DRG neurons. These findings support A3AR-based therapy as a viable approach to alleviate pain in different pathologies.
Conflict of interest statement
Conflict of interest statement
D.S. is a co-founder of BioIntervene, Inc, that licensed related intellectual property from Saint Louis University. The remaining authors have no conflicts of interest to declare.
Figures

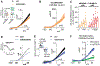
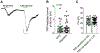


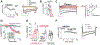
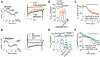

Similar articles
-
Acute visceral pain relief mediated by A3AR agonists in rats: involvement of N-type voltage-gated calcium channels.Pain. 2020 Sep 1;161(9):2179-2190. doi: 10.1097/j.pain.0000000000001905. Pain. 2020. PMID: 32379223 Free PMC article.
-
Covalently Binding Adenosine A3 Receptor Agonist ICBM Irreversibly Reduces Voltage-Gated Ca2+ Currents in Dorsal Root Ganglion Neurons.Purinergic Signal. 2024 Feb;20(1):35-45. doi: 10.1007/s11302-023-09929-y. Epub 2023 Mar 15. Purinergic Signal. 2024. PMID: 36918461 Free PMC article.
-
Uncovering the Mechanisms of Adenosine Receptor-Mediated Pain Control: Focus on the A3 Receptor Subtype.Int J Mol Sci. 2021 Jul 26;22(15):7952. doi: 10.3390/ijms22157952. Int J Mol Sci. 2021. PMID: 34360719 Free PMC article. Review.
-
Effects of synthetic A3 adenosine receptor agonists on cell proliferation and viability are receptor independent at micromolar concentrations.J Physiol Biochem. 2013 Sep;69(3):405-17. doi: 10.1007/s13105-012-0222-7. Epub 2012 Nov 27. J Physiol Biochem. 2013. PMID: 23184730
-
A3 Adenosine Receptors as Modulators of Inflammation: From Medicinal Chemistry to Therapy.Med Res Rev. 2018 Jul;38(4):1031-1072. doi: 10.1002/med.21456. Epub 2017 Jul 6. Med Res Rev. 2018. PMID: 28682469 Free PMC article. Review.
Cited by
-
Therapeutic Potential of Highly Selective A3 Adenosine Receptor Ligands in the Central and Peripheral Nervous System.Molecules. 2022 Mar 15;27(6):1890. doi: 10.3390/molecules27061890. Molecules. 2022. PMID: 35335254 Free PMC article. Review.
-
Adenosine signaling mediate pain transmission in the central nervous system.Purinergic Signal. 2023 Mar;19(1):245-254. doi: 10.1007/s11302-021-09826-2. Epub 2022 Jan 9. Purinergic Signal. 2023. PMID: 35000074 Free PMC article. Review.
-
Transient receptor potential ankyrin 1 contributes to somatic pain hypersensitivity in experimental colitis.Sci Rep. 2020 May 25;10(1):8632. doi: 10.1038/s41598-020-65618-5. Sci Rep. 2020. PMID: 32451393 Free PMC article.
-
The Anti-Inflammatory and Pain-Relieving Effects of AR170, an Adenosine A3 Receptor Agonist, in a Rat Model of Colitis.Cells. 2020 Jun 21;9(6):1509. doi: 10.3390/cells9061509. Cells. 2020. PMID: 32575844 Free PMC article.
-
Adenosine A3 receptor as a novel therapeutic target to reduce secondary events and improve neurocognitive functions following traumatic brain injury.J Neuroinflammation. 2020 Nov 12;17(1):339. doi: 10.1186/s12974-020-02009-7. J Neuroinflammation. 2020. PMID: 33183330 Free PMC article.
References
-
- Cai Q, Qiu CY, Qiu F, Liu TT, Qu ZW, Liu YM, Hu WP. Morphine inhibits acid-sensing ion channel currents in rat dorsal root ganglion neurons. Brain Res 2014;1554:12–20. - PubMed
-
- Coppi E, Maraula G, Fumagalli M, Failli P, Cellai L, Bonfanti E, Mazzoni L, Coppini R, Abbracchio MP, Pedata F, Pugliese AM. UDP-glucose enhances outward K plus currents necessary for cell differentiation and stimulates cell migration by activating the GPR17 receptor in oligodendrocyte precursors. Glia 2013;61:1155–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

