Cardiac implantable electronic device infections: Observational data from a tertiary care center in Lebanon
- PMID: 31008922
- PMCID: PMC6494368
- DOI: 10.1097/MD.0000000000014906
Cardiac implantable electronic device infections: Observational data from a tertiary care center in Lebanon
Abstract
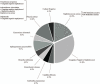
With increasing rates of device implantation, there is an increased recognition of device infection. We conducted a retrospective observational study in a tertiary care center in Lebanon, with data collected from medical records of patients presenting with cardiac implantable electronic device (CIED) infection from 2000 to 2017 with the purpose of identifying etiologies, risk factors and other parameters, and comparing them to available data from the rest of the world. We identified a total of 22 CIED infections. The most common microbial etiologies, including involvement in polymicrobial infection, were coagulase-negative staphylococci (45.5%) and Staphylococcus aureus (22.7%). Rare cases of Brucella melitensis, Sphingomonas paucimobilis, and Kytococcus schroeteri device infection were seen. Heart failure was seen in 77.3% of patients, hypertension in 68.2%, and chronic kidney disease in 50%. Skin changes were the most common presenting symptoms (86.4%). Antibiotics were given to all patients and all had their devices removed, with 36.4% undergoing new device implantation. This is the first study of CIED infections in Lebanon and the Middle East. Local epidemiology and occupational exposure must be considered while contemplating the microbial etiology of infection. Close monitoring after device implantation is important in preventing device infection that carries high risk of morbidity and mortality.
Figures

Similar articles
-
Staphylococcus aureus bacteraemia, cardiac implantable electronic device, extraction, and the risk of recurrent infection; a retrospective population-based cohort study.Infect Dis (Lond). 2024 Jul;56(7):543-553. doi: 10.1080/23744235.2024.2333444. Epub 2024 Mar 26. Infect Dis (Lond). 2024. PMID: 38529922
-
Clinical features and outcomes of cardiovascular implantable electronic device infections due to staphylococcal species.Am J Cardiol. 2012 Oct 15;110(8):1143-9. doi: 10.1016/j.amjcard.2012.05.052. Epub 2012 Jul 3. Am J Cardiol. 2012. PMID: 22762715
-
Redo procedures and chronic renal dysfunction are associated with higher risk of cardiac electronic device infections.Minerva Cardioangiol. 2018 Jun;66(3):225-232. doi: 10.23736/S0026-4725.18.04497-3. Epub 2018 Jan 5. Minerva Cardioangiol. 2018. PMID: 29308851
-
Contemporary management of cardiovascular implantable electronic device infections.Expert Rev Anti Infect Ther. 2010 Jul;8(7):831-9. doi: 10.1586/eri.10.54. Expert Rev Anti Infect Ther. 2010. PMID: 20586567 Review.
-
Cardiac implantable electronic device infections: the enemy that lurks beneath the skin.J Long Term Eff Med Implants. 2010;20(3):203-17. doi: 10.1615/jlongtermeffmedimplants.v20.i3.40. J Long Term Eff Med Implants. 2010. PMID: 21395519 Review.
Cited by
-
Prevention of Cardiac Implantable Electronic Device Infections: A Review.Rev Cardiovasc Med. 2023 Jun 14;24(6):176. doi: 10.31083/j.rcm2406176. eCollection 2023 Jun. Rev Cardiovasc Med. 2023. PMID: 39077520 Free PMC article. Review.
-
Postimplantation pocket hematoma increases risk of cardiac implantable electronic device infection: A meta-analysis.J Arrhythm. 2021 Mar 13;37(3):635-644. doi: 10.1002/joa3.12516. eCollection 2021 Jun. J Arrhythm. 2021. PMID: 34141016 Free PMC article.
-
The Gram-Negative Bacilli Isolated from Caves-Sphingomonas paucimobilis and Hafnia alvei and a Review of Their Involvement in Human Infections.Int J Environ Res Public Health. 2022 Feb 17;19(4):2324. doi: 10.3390/ijerph19042324. Int J Environ Res Public Health. 2022. PMID: 35206510 Free PMC article. Review.
-
Brucella cardiac implantable electronic device infection: A single-center case series.Ann Med Surg (Lond). 2021 Jul 16;68:102568. doi: 10.1016/j.amsu.2021.102568. eCollection 2021 Aug. Ann Med Surg (Lond). 2021. PMID: 34367635 Free PMC article.
-
A case of Kytococcus schroeteri prosthetic valve endocarditis in a patient with COVID-19 infection.Indian J Med Microbiol. 2023 Mar-Apr;42:89-91. doi: 10.1016/j.ijmmb.2022.09.001. Epub 2022 Sep 26. Indian J Med Microbiol. 2023. PMID: 36175197 Free PMC article.
References
-
- Gould PA, Gula LJ, Yee R, et al. Cardiovascular implantable electrophysiological device-related infections: a review. Curr Opin Cardiol 2011;26:6–11. - PubMed
-
- Greenspon AJ, Patel JD, Lau E, et al. 16-year trends in the infection burden for pacemakers and implantable cardioverter-defibrillators in the United States 1993 to 2008. J Am Coll Cardiol 2011;58:1001–6. - PubMed
-
- Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010;121:458–77. - PubMed
-
- Karchmer AW. Infections involving cardiac implantable electronic devices. UpToDate, 2017, edited by Ted. W. Post, published by UpToDate in Waltham, MA.
-
- Sridhar AR, Lavu M, Yarlagadda V, et al. Cardiac implantable electronic device-related infection and extraction trends in the U.S. PACE 2017;40:286–93. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

