Diagnostic performance of cytomegalovirus (CMV) immune monitoring with ELISPOT and QuantiFERON-CMV assay in kidney transplantation: A PRISMA-compliant article
- PMID: 31008952
- PMCID: PMC6494277
- DOI: 10.1097/MD.0000000000015228
Diagnostic performance of cytomegalovirus (CMV) immune monitoring with ELISPOT and QuantiFERON-CMV assay in kidney transplantation: A PRISMA-compliant article
Abstract
Background: Cytomegalovirus (CMV) infection is part of major infection complications following kidney transplantation. However, more rapid and low-complexity assays are needed for CMV infection. Our study is to investigate the diagnostic efficacy of 2 novel tests, CMV-ELISPOT and QuantiFERON-CMV tests, in CMV DNA viremia and CMV infection following renal transplant.
Methods: We searched MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials and the Web of Science. Case-control or cohort study designed to explore the CMV-ELISPOT and/or QuantiFERON-CMV tests in the recipients with CMV infection was considered to be eligible for this study. Sensitivity (SEN), specificity (SPE), diagnostic odds ratio (DOR), and summary receiver-operating characteristic (SROC) curves were calculated.
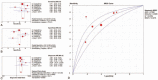
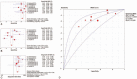
Results: We selected a total of 12 articles for systematic review and 11 of them were included in meta-analysis. For CMV-pp65 assay, the pooled SEN, SPE, and DOR were 0.73 (95% confidence interval [CI], 0.67-0.78), 0.61 (95% CI, 0.56-0.65), and 4.46 (95% CI, 3.11-6.39), respectively. For CMV-IE-1 assay, the pooled SEN, SPE, and DOR were 0.84 (95% CI, 0.78-0.88), 0.46 (95% CI, 0.42-0.51), and 5.07 (95% CI, 3.26-7.89), respectively, whereas the pooled SEN, SPE, and DOR of QuantiFERON-CMV test were 0.38 (95% CI, 0.28-0.49), 0.38 (95% CI, 0.32-0.44), and 1.02 (95% CI, 0.17-6.00).
Conclusions: We reported that CMV-ELISPOT tests, including CMV-pp65 and CMV-IE-1, perform well in the diagnosis and prediction of CMV infection in renal transplant recipients, whereas QuantiFERON-CMV test needs further exploration.
Figures


References
-
- Noble J, Gatault P, Sautenet B, et al. Predictive factors of spontaneous CMV DNAemia clearance in kidney transplantation. J Clin Virol 2017;99-100:38–43. - PubMed
-
- Fishman JA, Emery V, Freeman R, et al. Cytomegalovirus in transplantation—challenging the status quo. Clin Transplan 2007;21:149–58. - PubMed
-
- Fishman JA. Infection in renal transplant recipients. Semin Nephrol 2007;27:445–61. - PubMed
-
- Humar A, Michaels M. Monitoring AIWGoID. American Society of Transplantation recommendations for screening, monitoring and reporting of infectious complications in immunosuppression trials in recipients of organ transplantation. Am J Transplant 2006;6:262–74. - PubMed
-
- Hodson EM, Ladhani M, Webster AC, et al. Antiviral medications for preventing cytomegalovirus disease in solid organ transplant recipients. Cochrane Database Syst Rev 2013;2: CD003774. - PubMed

