Selection of adjuvants for vaccines targeting specific pathogens
- PMID: 31009255
- PMCID: PMC7103699
- DOI: 10.1080/14760584.2019.1604231
Selection of adjuvants for vaccines targeting specific pathogens
Abstract
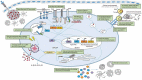
Adjuvants form an integral component in most of the inactivated and subunit vaccine formulations. Careful and proper selection of adjuvants helps in promoting appropriate immune responses against target pathogens at both innate and adaptive levels such that protective immunity can be elicited. Areas covered: Herein, we describe the recent progress in our understanding of the mode of action of adjuvants that are licensed for use in human vaccines or in clinical or pre-clinical stages at both innate and adaptive levels. Different pathogens have distinct characteristics, which require the host to mount an appropriate immune response against them. Adjuvants can be selected to elicit a tailor-made immune response to specific pathogens based on their unique properties. Identification of biomarkers of adjuvanticity for several candidate vaccines using omics-based technologies can unravel the mechanism of action of modern and experimental adjuvants. Expert opinion: Adjuvant technology has been revolutionized over the last two decades. In-depth understanding of the role of adjuvants in activating the innate immune system, combined with systems vaccinology approaches, have led to the development of next-generation, novel adjuvants that can be used in vaccines against challenging pathogens and in specific target populations.
Keywords: Adjuvants; immunity; mechanism of action; pathogen; systems vaccinology.
Figures
References
-
- Roush SW, Murphy TV.. Vaccine-Preventable Disease Table Working Group. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA. 2007;298(18):2155–2163. - PubMed
-
- Garcia A, De Sanctis JB. An overview of adjuvant formulations and delivery systems. APMIS. 2014;122(4):257–267. - PubMed
-
- Henriksen-Lacey M, Korsholm KS, Andersen P, et al. Liposomal vaccine delivery systems. Expert Opin Drug Deliv. 2011;8(4):505–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
