CD28 Superagonistic Activation of T Cells Induces a Tumor Cell-Like Metabolic Program
- PMID: 31009338
- PMCID: PMC6634261
- DOI: 10.1089/mab.2018.0042
CD28 Superagonistic Activation of T Cells Induces a Tumor Cell-Like Metabolic Program
Abstract
CD28 superagonist (CD28SA), a therapeutic immunomodulatory monoclonal antibody triggered rapid and exaggerated activation of CD4+ effector memory T cells (TEMs) in humans with unwanted serious adverse effects. It is well known that distinct metabolic programs determine the fate and responses of immune cells. In this study, we show that human CD4+ TEMs stimulated with CD28SA adopt a metabolic program similar to those of tumor cells with enhanced glucose utilization, lipid biosynthesis, and proliferation in hypoxic conditions. Identification of metabolic profiles underlying hyperactive T cell activation would provide a platform to test safety of immunostimulatory antibodies.
Keywords: CD28 superagonist; CD4 effector memory T cells; glycolysis; lipogenesis; oxidative phosphorylation.
Conflict of interest statement
No competing financial interests exist.
Figures
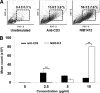

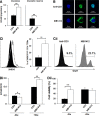

References
-
- Tabares P, Berr S, Romer PS, Chuvpilo S, Matskevich AA, Tyrsin D, Fedotov Y, Einsele H, Tony HP, and Hunig T: Human regulatory T cells are selectively activated by low-dose application of the CD28 superagonist TGN1412/TAB08. Eur J Immunol 2014;44:1225–1236 - PubMed
-
- Suntharalingam G, Perry MR, Ward S, Brett SJ, Castello-Cortes A, Brunner MD, and Panoskaltsis N: Cytokine storm in a phase 1 trial of the anti-CD28 monoclonal antibody TGN1412. N Engl J Med 2006;355:1018–1028 - PubMed
-
- Mirenda V, Jarmin SJ, David R, Dyson J, Scott D, Gu Y, Lechler RI, Okkenhaug K, and Marelli-Berg FM: Physiologic and aberrant regulation of memory T-cell trafficking by the costimulatory molecule CD28. Blood 2007;109:2968–2977 - PubMed
-
- Thaventhiran T, Alhumeed N, Yeang HX, Sethu S, Downey JS, Alghanem AF, Olayanju A, Smith EL, Cross MJ, Webb SD, Williams DP, Bristow A, Ball C, Stebbings R, and Sathish JG: Failure to upregulate cell surface PD-1 is associated with dysregulated stimulation of T cells by TGN1412-like CD28 superagonist. MAbs 2014;6:1290–1299 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
