Neural crest streaming as an emergent property of tissue interactions during morphogenesis
- PMID: 31009457
- PMCID: PMC6497294
- DOI: 10.1371/journal.pcbi.1007002
Neural crest streaming as an emergent property of tissue interactions during morphogenesis
Abstract
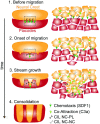
A fundamental question in embryo morphogenesis is how a complex pattern is established in seemingly uniform tissues. During vertebrate development, neural crest cells differentiate as a continuous mass of tissue along the neural tube and subsequently split into spatially distinct migratory streams to invade the rest of the embryo. How these streams are established is not well understood. Inhibitory signals surrounding the migratory streams led to the idea that position and size of streams are determined by a pre-pattern of such signals. While clear evidence for a pre-pattern in the cranial region is still lacking, all computational models of neural crest migration published so far have assumed a pre-pattern of negative signals that channel the neural crest into streams. Here we test the hypothesis that instead of following a pre-existing pattern, the cranial neural crest creates their own migratory pathway by interacting with the surrounding tissue. By combining theoretical modeling with experimentation, we show that streams emerge from the interaction of the hindbrain neural crest and the neighboring epibranchial placodal tissues, without the need for a pre-existing guidance cue. Our model suggests that the initial collective neural crest invasion is based on short-range repulsion and asymmetric attraction between neighboring tissues. The model provides a coherent explanation for the formation of cranial neural crest streams in concert with previously reported findings and our new in vivo observations. Our results point to a general mechanism of inducing collective invasion patterns.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways.Development. 2002 Jan;129(2):433-42. doi: 10.1242/dev.129.2.433. Development. 2002. PMID: 11807035
-
In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches.Development. 2000 Mar;127(6):1161-72. doi: 10.1242/dev.127.6.1161. Development. 2000. PMID: 10683170
-
Cranial neural crest migration: new rules for an old road.Dev Biol. 2010 Aug 15;344(2):543-54. doi: 10.1016/j.ydbio.2010.04.010. Epub 2010 Apr 23. Dev Biol. 2010. PMID: 20399765 Free PMC article. Review.
-
Combined intrinsic and extrinsic influences pattern cranial neural crest migration and pharyngeal arch morphogenesis in axolotl.Dev Biol. 2004 Feb 15;266(2):252-69. doi: 10.1016/j.ydbio.2003.09.039. Dev Biol. 2004. PMID: 14738875
-
Neural crest cell formation and migration in the developing embryo.FASEB J. 1994 Jul;8(10):699-706. doi: 10.1096/fasebj.8.10.8050668. FASEB J. 1994. PMID: 8050668 Review.
Cited by
-
Disturbances of the stomatognathic system and possibilities of its correction in patients with craniofacial morphea.Postepy Dermatol Alergol. 2023 Oct;40(5):592-598. doi: 10.5114/ada.2023.131865. Epub 2023 Nov 9. Postepy Dermatol Alergol. 2023. PMID: 38028421 Free PMC article. Review.
-
Paracrine regulation of neural crest EMT by placodal MMP28.PLoS Biol. 2023 Aug 17;21(8):e3002261. doi: 10.1371/journal.pbio.3002261. eCollection 2023 Aug. PLoS Biol. 2023. PMID: 37590318 Free PMC article.
-
Dynamic fibronectin assembly and remodeling by leader neural crest cells prevents jamming in collective cell migration.Elife. 2023 Apr 19;12:e83792. doi: 10.7554/eLife.83792. Elife. 2023. PMID: 37073859 Free PMC article.
-
Evolutionary and Developmental Associations of Neural Crest and Placodes in the Vertebrate Head: Insights From Jawless Vertebrates.Front Physiol. 2020 Aug 13;11:986. doi: 10.3389/fphys.2020.00986. eCollection 2020. Front Physiol. 2020. PMID: 32903576 Free PMC article. Review.
-
Overview of Head Muscles with Special Emphasis on Extraocular Muscle Development.Adv Anat Embryol Cell Biol. 2023;236:57-80. doi: 10.1007/978-3-031-38215-4_3. Adv Anat Embryol Cell Biol. 2023. PMID: 37955771
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

