Clumping factor B is an important virulence factor during Staphylococcus aureus skin infection and a promising vaccine target
- PMID: 31009507
- PMCID: PMC6497315
- DOI: 10.1371/journal.ppat.1007713
Clumping factor B is an important virulence factor during Staphylococcus aureus skin infection and a promising vaccine target
Abstract
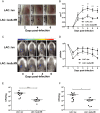
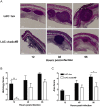
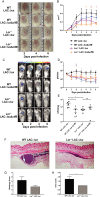
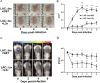
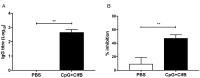
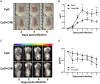
Staphylococcus aureus expresses a number of cell wall-anchored proteins that mediate adhesion and invasion of host cells and tissues and promote immune evasion, consequently contributing to the virulence of this organism. The cell wall-anchored protein clumping factor B (ClfB) has previously been shown to facilitate S. aureus nasal colonization through high affinity interactions with the cornified envelope in the anterior nares. However, the role of ClfB during skin and soft tissue infection (SSTI) has never been investigated. This study reveals a novel role for ClfB during SSTIs. ClfB is crucial in determining the abscess structure and bacterial burden early in infection and this is dependent upon a specific interaction with the ligand loricrin which is expressed within the abscess tissue. Targeting ClfB using a model vaccine that induced both protective humoral and cellular responses, leads to protection during S. aureus skin infection. This study therefore identifies ClfB as an important antigen for future SSTI vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Nasal colonisation by Staphylococcus aureus depends upon clumping factor B binding to the squamous epithelial cell envelope protein loricrin.PLoS Pathog. 2012 Dec;8(12):e1003092. doi: 10.1371/journal.ppat.1003092. Epub 2012 Dec 27. PLoS Pathog. 2012. PMID: 23300445 Free PMC article.
-
Force-Induced Strengthening of the Interaction between Staphylococcus aureus Clumping Factor B and Loricrin.mBio. 2017 Dec 5;8(6):e01748-17. doi: 10.1128/mBio.01748-17. mBio. 2017. PMID: 29208742 Free PMC article.
-
Clumping Factor B Promotes Adherence of Staphylococcus aureus to Corneocytes in Atopic Dermatitis.Infect Immun. 2017 May 23;85(6):e00994-16. doi: 10.1128/IAI.00994-16. Print 2017 Jun. Infect Immun. 2017. PMID: 28373353 Free PMC article.
-
Host-pathogen interactions between the skin and Staphylococcus aureus.Curr Opin Microbiol. 2012 Feb;15(1):28-35. doi: 10.1016/j.mib.2011.11.003. Epub 2011 Dec 1. Curr Opin Microbiol. 2012. PMID: 22137885 Free PMC article. Review.
-
A play in four acts: Staphylococcus aureus abscess formation.Trends Microbiol. 2011 May;19(5):225-32. doi: 10.1016/j.tim.2011.01.007. Epub 2011 Feb 25. Trends Microbiol. 2011. PMID: 21353779 Free PMC article. Review.
Cited by
-
Functional diversity of staphylococcal surface proteins at the host-microbe interface.Front Microbiol. 2023 May 18;14:1196957. doi: 10.3389/fmicb.2023.1196957. eCollection 2023. Front Microbiol. 2023. PMID: 37275142 Free PMC article. Review.
-
Immunoinformatic prediction to identify Staphylococcus aureus peptides that bind to CD8+ T-cells as potential vaccine candidates.Vet World. 2024 Jun;17(6):1413-1422. doi: 10.14202/vetworld.2024.1413-1422. Epub 2024 Jun 28. Vet World. 2024. PMID: 39077442 Free PMC article.
-
IL-10 inhibition during immunization improves vaccine-induced protection against Staphylococcus aureus infection.JCI Insight. 2024 May 28;9(13):e178216. doi: 10.1172/jci.insight.178216. JCI Insight. 2024. PMID: 38973612 Free PMC article.
-
Annexin A2-Mediated Internalization of Staphylococcus aureus into Bovine Mammary Epithelial Cells Requires Its Interaction with Clumping Factor B.Microorganisms. 2021 Oct 3;9(10):2090. doi: 10.3390/microorganisms9102090. Microorganisms. 2021. PMID: 34683411 Free PMC article.
-
Clonal Spreading of ST42 Staphylococcus haemolyticus Strains Occurs Possibly Due to fusB and tetK Resistant Genes and Capsule-Related Genes.Int J Mol Sci. 2023 Mar 24;24(7):6198. doi: 10.3390/ijms24076198. Int J Mol Sci. 2023. PMID: 37047168 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

