Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage
- PMID: 31009511
- PMCID: PMC6497319
- DOI: 10.1371/journal.ppat.1007625
Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage
Abstract
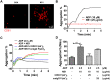
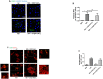
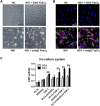
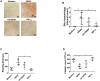
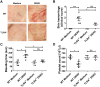
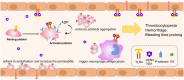
Dengue virus (DENV) infection, the most common mosquito-transmitted viral infection, can cause a range of diseases from self-limiting dengue fever to life-threatening dengue hemorrhagic fever and shock syndrome. Thrombocytopenia is a major characteristic observed in both mild and severe dengue disease and is significantly correlated with the progression of dengue severity. Previous studies have shown that DENV nonstructural protein 1 (NS1), which can be secreted into patients' blood, can stimulate immune cells via Toll-like receptor 4 (TLR4) and can cause endothelial leakage. However, it is unclear whether DENV NS1 can directly induce platelet activation or cause thrombocytopenia during DENV infection. In this study, we first demonstrated that DENV but not Zika virus cell culture supernatant could induce P-selectin expression and phosphatidylserine (PS) exposure in human platelets, both of which were abolished when NS1 was depleted from the DENV supernatant. Similar results were found using recombinant NS1 from all four serotypes of DENV, and those effects were blocked in the presence of anti-NS1 F(ab')2, anti-TLR4 antibody, a TLR4 antagonist (Rhodobacter sphaeroides lipopolysaccharide, LPS-Rs) and a TLR4 signaling inhibitor (TAK242), but not polymyxin B (an LPS inhibitor). Moreover, the activation of platelets by DENV NS1 promoted subthreshold concentrations of adenosine diphosphate (ADP)-induced platelet aggregation and enhanced platelet adhesion to endothelial cells and phagocytosis by macrophages. Finally, we demonstrated that DENV-induced thrombocytopenia and hemorrhage were attenuated in TLR4 knockout and wild-type mice when NS1 was depleted from DENV supernatant. Taken together, these results suggest that the binding of DENV NS1 to TLR4 on platelets can trigger its activation, which may contribute to thrombocytopenia and hemorrhage during dengue infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Anti-dengue virus nonstructural protein 1 antibodies contribute to platelet phagocytosis by macrophages.Thromb Haemost. 2016 Mar;115(3):646-56. doi: 10.1160/TH15-06-0498. Epub 2015 Dec 3. Thromb Haemost. 2016. PMID: 26632672
-
Therapeutic Effects of Monoclonal Antibody against Dengue Virus NS1 in a STAT1 Knockout Mouse Model of Dengue Infection.J Immunol. 2017 Oct 15;199(8):2834-2844. doi: 10.4049/jimmunol.1601523. Epub 2017 Sep 13. J Immunol. 2017. PMID: 28904127
-
Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components.PLoS Pathog. 2017 Nov 9;13(11):e1006673. doi: 10.1371/journal.ppat.1006673. eCollection 2017 Nov. PLoS Pathog. 2017. PMID: 29121099 Free PMC article.
-
Re-evaluation of the pathogenic roles of nonstructural protein 1 and its antibodies during dengue virus infection.J Biomed Sci. 2013 Jun 27;20(1):42. doi: 10.1186/1423-0127-20-42. J Biomed Sci. 2013. PMID: 23806052 Free PMC article. Review.
-
Dengue virus non-structural protein 1: a pathogenic factor, therapeutic target, and vaccine candidate.J Biomed Sci. 2018 Jul 24;25(1):58. doi: 10.1186/s12929-018-0462-0. J Biomed Sci. 2018. PMID: 30037331 Free PMC article. Review.
Cited by
-
Expansion and Refinement of Deep Sequence-Coupled Biopanning Technology for Epitope-Specific Antibody Responses in Human Serum.Viruses. 2020 Sep 30;12(10):1114. doi: 10.3390/v12101114. Viruses. 2020. PMID: 33008118 Free PMC article.
-
Dengue mouse models for evaluating pathogenesis and countermeasures.Curr Opin Virol. 2020 Aug;43:50-58. doi: 10.1016/j.coviro.2020.09.001. Epub 2020 Sep 17. Curr Opin Virol. 2020. PMID: 32950933 Free PMC article. Review.
-
Hyperinflammation, apoptosis, and organ damage.Exp Biol Med (Maywood). 2022 Jul;247(13):1112-1123. doi: 10.1177/15353702221090454. Epub 2022 Apr 27. Exp Biol Med (Maywood). 2022. PMID: 35475359 Free PMC article. Review.
-
Identification and verification of differentially expressed key genes in peripheral blood-derived T cells between chronic immune thrombocytopenia patients and healthy controls.Bioengineered. 2022 May;13(5):13587-13595. doi: 10.1080/21655979.2022.2080422. Bioengineered. 2022. PMID: 35796625 Free PMC article.
-
Viruses and thrombocytopenia.Heliyon. 2024 Mar 16;10(6):e27844. doi: 10.1016/j.heliyon.2024.e27844. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524607 Free PMC article. Review.
References
-
- WorldHealthOrganization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. WHO Guidelines Approved by the Guidelines Review Committee. 2009. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

