Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection
- PMID: 31009520
- PMCID: PMC6497318
- DOI: 10.1371/journal.ppat.1007717
Candida albicans induces mucosal bacterial dysbiosis that promotes invasive infection
Abstract
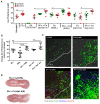
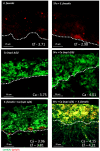
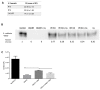
Infectious complications are a common cause of morbidity and mortality in cancer patients undergoing chemotherapy due to increased risk of oral and gastrointestinal candidiasis, candidemia and septicemia. Interactions between C. albicans and endogenous mucosal bacteria are important in understanding the mechanisms of invasive infection. We published a mouse intravenous chemotherapy model that recapitulates oral and intestinal mucositis, and myelosuppression in patients receiving 5-fluorouracil. We used this model to study the influence of C. albicans on the mucosal bacterial microbiome and compared global community changes in the oral and intestinal mucosa of the same mice. We validated 16S rRNA gene sequencing data by qPCR, in situ hybridization and culture approaches. Mice receiving both 5Fu and C. albicans had an endogenous bacterial overgrowth on the oral but not the small intestinal mucosa. C. albicans infection was associated with loss of mucosal bacterial diversity in both sites with indigenous Stenotrophomonas, Alphaproteobacteria and Enterococcus species dominating the small intestinal, and Enterococcus species dominating the oral mucosa. Both immunosuppression and Candida infection contributed to changes in the oral microbiota. Enterococci isolated from mice with oropharyngeal candidiasis were implicated in degrading the epithelial junction protein E-cadherin and increasing the permeability of the oral epithelial barrier in vitro. Importantly, depletion of these organisms with antibiotics in vivo attenuated oral mucosal E-cadherin degradation and C. albicans invasion without affecting fungal burdens, indicating that bacterial community changes represent overt dysbiosis. Our studies demonstrate a complex interaction between C. albicans, the resident mucosal bacterial microbiota and the host environment in pathogenesis. We shed significant new light on the role of C. albicans in shaping resident bacterial communities and driving mucosal dysbiosis.
Conflict of interest statement
N/A
Figures






References
-
- Lalla RV, Latortue MC, Hong CH, Ariyawardana A, D'Amato-Palumbo S, Fischer DJ, Martof A, Nicolatou-Galitis O, Patton LL, Elting LS, Spijkervet FK, Brennan MT; Fungal Infections Section, Oral Care Study Group, Multinational Association of Supportive Care in Cancer (MASCC)/International Society of Oral Oncology (ISOO). A systematic review of oral fungal infections in patients receiving cancer therapy. Support Care Cancer. 2010. August;18(8):985–92. 10.1007/s00520-010-0892-z - DOI - PMC - PubMed
-
- Peterson DE, Boers-Doets CB, Bensadoun RJ, Herrstedt J; ESMO Guidelines Committee. Management of oral and gastrointestinal mucosal injury: ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 2015. September;26 Suppl 5:v139–51. 10.1093/annonc/mdv202 Epub 2015 Jul 4. - DOI - PubMed
-
- Cole GT, Halawa AA, Anaissie EJ. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1996;22 Suppl 2:S73–88. - PubMed
-
- Jarvis WR. Epidemiology of nosocomial fungal infections, with emphasis on Candida species. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 1995;20(6):1526–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

