Leber Congenital Amaurosis (LCA): Potential for Improvement of Vision
- PMID: 31009524
- PMCID: PMC6892385
- DOI: 10.1167/iovs.19-26672
Leber Congenital Amaurosis (LCA): Potential for Improvement of Vision
Figures
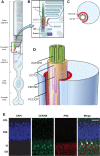
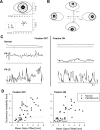
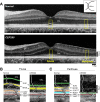
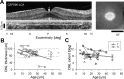


Similar articles
-
The genetic profile of Leber congenital amaurosis in an Australian cohort.Mol Genet Genomic Med. 2017 Nov;5(6):652-667. doi: 10.1002/mgg3.321. Epub 2017 Aug 22. Mol Genet Genomic Med. 2017. PMID: 29178642 Free PMC article.
-
Visual acuity in patients with Leber's congenital amaurosis and early childhood-onset retinitis pigmentosa.Ophthalmology. 2010 Jun;117(6):1190-8. doi: 10.1016/j.ophtha.2009.09.056. Epub 2010 Jan 15. Ophthalmology. 2010. PMID: 20079931
-
Copy number variations and multiallelic variants in Korean patients with Leber congenital amaurosis.Mol Vis. 2020 Feb 24;26:26-35. eCollection 2020. Mol Vis. 2020. PMID: 32165824 Free PMC article.
-
A Mini-review: Animal Models of GUCY2D Leber Congenital Amaurosis (LCA1).Adv Exp Med Biol. 2016;854:253-8. doi: 10.1007/978-3-319-17121-0_34. Adv Exp Med Biol. 2016. PMID: 26427419 Review.
-
A Mini-Review: Leber Congenital Amaurosis: Identification of Disease-Causing Variants and Personalised Therapies.Adv Exp Med Biol. 2018;1074:265-271. doi: 10.1007/978-3-319-75402-4_32. Adv Exp Med Biol. 2018. PMID: 29721952 Review.
Cited by
-
Photoreceptor function and structure in retinal degenerations caused by biallelic BEST1 mutations.Vision Res. 2023 Feb;203:108157. doi: 10.1016/j.visres.2022.108157. Epub 2022 Nov 28. Vision Res. 2023. PMID: 36450205 Free PMC article.
-
Treatment Potential for Macular Cone Vision in Leber Congenital Amaurosis Due to CEP290 or NPHP5 Mutations: Predictions From Artificial Intelligence.Invest Ophthalmol Vis Sci. 2019 Jun 3;60(7):2551-2562. doi: 10.1167/iovs.19-27156. Invest Ophthalmol Vis Sci. 2019. PMID: 31212307 Free PMC article.
-
Pathways and disease-causing alterations in visual chromophore production for vertebrate vision.J Biol Chem. 2021 Jan-Jun;296:100072. doi: 10.1074/jbc.REV120.014405. Epub 2020 Nov 23. J Biol Chem. 2021. PMID: 33187985 Free PMC article. Review.
-
Retinal response to light exposure in BEST1-mutant dogs evaluated with ultra-high resolution OCT.Vision Res. 2024 May;218:108379. doi: 10.1016/j.visres.2024.108379. Epub 2024 Mar 8. Vision Res. 2024. PMID: 38460402 Free PMC article.
-
Gene regulatory and gene editing tools and their applications for retinal diseases and neuroprotection: From proof-of-concept to clinical trial.Front Neurosci. 2022 Oct 20;16:924917. doi: 10.3389/fnins.2022.924917. eCollection 2022. Front Neurosci. 2022. PMID: 36340792 Free PMC article. Review.
References
-
- Bramall AN, Wright AF, Jacobson SG, McInnes RR. The genomic, biochemical, and cellular responses of the retina in inherited photoreceptor degenerations and prospects for the treatment of these disorders. Annu Rev Neurosci. 2010;33:441–472. - PubMed
-
- Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11:273–284. - PubMed
-
- Haider NB, Jacobson SG, Cideciyan AV, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24:127–131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

