Synthesis of Structurally Diverse 3-, 4-, 5-, and 6-Membered Heterocycles from Diisopropyl Iminomalonates and Soft C-Nucleophiles
- PMID: 31009563
- PMCID: PMC7879484
- DOI: 10.1021/acs.joc.9b00681
Synthesis of Structurally Diverse 3-, 4-, 5-, and 6-Membered Heterocycles from Diisopropyl Iminomalonates and Soft C-Nucleophiles
Abstract
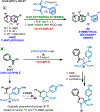
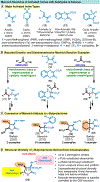
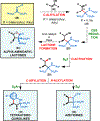
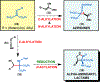
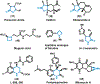
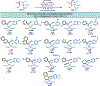
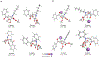
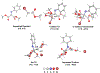
Herein, we present a general synthetic strategy for the preparation of 3-, 4-, 5-, and 6-membered heterocyclic unnatural amino acid derivatives by exploiting facile Mannich-type reactions between readily available N-alkyl- and N-aryl-substituted diisopropyl iminomalonates and a wide range of soft anionic C-nucleophiles without using any catalyst or additive. Fully substituted aziridines were obtained in a single step when enolates of α-bromo esters were employed as nucleophiles. Enantiomerically enriched azetidines, γ-lactones, and tetrahydroquinolines were obtained via a two-step catalytic asymmetric reduction and cyclization sequence from ketone enolate-derived adducts. Finally, highly substituted γ-lactams were prepared in one pot from adducts obtained using acetonitrile-derived carbanions. Overall, this work clearly demonstrates the utility of iminomalonates as highly versatile building blocks for the practical and scalable synthesis of structurally diverse heterocycles.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



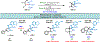


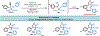
Similar articles
-
Catalytic Asymmetric Reactions with N-Metallated Azomethine Ylides.Acc Chem Res. 2020 May 19;53(5):1084-1100. doi: 10.1021/acs.accounts.0c00113. Epub 2020 Apr 22. Acc Chem Res. 2020. PMID: 32320206
-
From racemic precursors to fully stereocontrolled products: one-pot synthesis of chiral α-amino lactones and lactams.Org Biomol Chem. 2016 Jul 14;14(26):6316-27. doi: 10.1039/c6ob00953k. Epub 2016 Jun 7. Org Biomol Chem. 2016. PMID: 27270561
-
Catalytically Generated Vanadium Enolates Formed via Interruption of the Meyer-Schuster Rearrangement as Useful Reactive Intermediates.Acc Chem Res. 2020 Aug 18;53(8):1568-1579. doi: 10.1021/acs.accounts.0c00285. Epub 2020 Jul 21. Acc Chem Res. 2020. PMID: 32692147
-
Configurationally labile α-bromoacid derivatives for asymmetric preparation of heterocycles.Org Biomol Chem. 2025 May 21;23(20):4828-4845. doi: 10.1039/d5ob00207a. Org Biomol Chem. 2025. PMID: 40266563 Review.
-
[Efficient chiral control based on five-membered heterocyclic and related systems].Yakugaku Zasshi. 2000 Dec;120(12):1323-35. doi: 10.1248/yakushi1947.120.12_1323. Yakugaku Zasshi. 2000. PMID: 11193382 Review. Japanese.
Cited by
-
Total synthesis of isatindigotindoline C.Org Biomol Chem. 2020 Mar 18;18(11):2051-2053. doi: 10.1039/d0ob00270d. Org Biomol Chem. 2020. PMID: 32141462 Free PMC article.
-
Aza-Quasi-Favorskii Reaction: Construction of Highly Substituted Aziridines through a Concerted Multibond Rearrangement Process.J Am Chem Soc. 2022 Jun 22;144(24):10943-10949. doi: 10.1021/jacs.2c03805. Epub 2022 Jun 8. J Am Chem Soc. 2022. PMID: 35674783 Free PMC article.
-
Practical asymmetric amine nucleophilic approach for the modular construction of protected α-quaternary amino acids.Chem Sci. 2022 Jun 5;13(23):6806-6812. doi: 10.1039/d2sc02318k. eCollection 2022 Jun 15. Chem Sci. 2022. PMID: 35774153 Free PMC article.
References
-
- Kattamuri PV; Yin J; Siriwongsup S; Kwon D-H; Ess DH; Li Q; Li G; Yousufuddin M; Richardson PF; Sutton SC; Kürti L, Practical Singly and Doubly Electrophilic Aminating Agents: A New, More Sustainable Platform for Carbon–Nitrogen Bond Formation. J. Am. Chem. Soc 2017, 139, 11184–11196; - PubMed
- Kattamuri PV; Yin J; Siriwongsup S; Kwon D-H; Ess DH; Li Q; Li G; Yousufuddin M; Richardson PF; Sutton SC; Kürti L, Correction to “Practical Singly and Doubly Electrophilic Aminating Agents: A New, More Sustainable Platform for Carbon–Nitrogen Bond Formation”. J. Am. Chem. Soc 2019, 141, 3315–3315. - PubMed
-
- Stevenazzi A; Marchini M; Sandrone G; Vergani B; Lattanzio M, Amino acidic scaffolds bearing unnatural side chains: An old idea generates new and versatile tools for the life sciences. Bioorg. Med. Chem. Lett 2014, 24, 5349–5356; - PubMed
- Blaskovich MAT, Unusual Amino Acids in Medicinal Chemistry. J. Med. Chem 2016, 59, 10807–10836; - PubMed
- Saghyan Ashot S. and Langer Peter Eds. Asymmetric Synthesis of Nonproteinogenic Amino Acids. (Wiley-VCH, Weinheim, Germany, 2016);
- Ni S; Garrido-Castro AF; Merchant RR; de Gruyter JN; Schmitt DC; Mousseau JJ; Gallego GM; Yang S; Collins MR; Qiao JX; Yeung K-S; Langley DR; Poss MA; Scola PM; Qin T; Baran PS, A General Amino Acid Synthesis Enabled by Innate Radical Cross-Coupling. Angew. Chem. Int. Ed 2018, 57, 14560–14565. - PMC - PubMed
-
- Mao B; Fañanás-Mastral M; Feringa BL, Catalytic Asymmetric Synthesis of Butenolides and Butyrolactones. Chem. Rev 2017, 117, 10502–10566; - PMC - PubMed
- Murauski KJR; Jaworski AA; Scheidt KA, A continuing challenge: N-heterocyclic carbene-catalyzed syntheses of γ-butyrolactones. Chem. Soc. Rev 2018, 47, 1773–1782. - PubMed
-
- Burns NZ; Jacobsen EN Mannich Reaction In Science of Synthesis: Stereoselective Synthesis 2: Stereoselective Reactions of Carbonyl and Imino Groups; Molander G, Ed.; Georg Thieme Verlag: New York, 2010; Chapter 2.16.
-
- Saranya S; Harry NA; Krishnan KK; Anilkumar G, Recent Developments and Perspectives in the Asymmetric Mannich Reaction. Asian J. Org. Chem 2018, 7, 613–633 and references cited therein.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

